COVID-19 announcements
Catch up on news and announcements regarding the COVID-19 vaccine. Latest news about vaccines will be featured on the Vaccine Hub homepage.
October 2024
From 1 October 2024 RATs are available to purchase from community pharmacies and retail shops
From 1 October 2024, if you need RATs, you can buy them from community pharmacies and retail shops such as supermarkets.
If you meet Pharmac’s eligibility criteria, you will still be able to access COVID-19 antiviral treatment medicine through your healthcare provider and pharmacy. Please get yourself free RATs while they are available https://info.health.nz/.../covid-19/antiviral-medicines...
Public health advice for the management of COVID-19 remains unchanged https://info.health.nz/condi.../infectious-diseases/covid-19
Being up to date with your COVID-19 vaccinations remains one of the best things we can do to protect ourselves from serious illness, hospitalisation, and death from COVID-19. You can book for yourself, a family member, or a group on bookmyvaccine.health.nz
You can find out more about this change on the Health New Zealand | Te Whatu Ora website
March 2024
Changes to COVID-19 testing advice for household contacts - 4 March 2024
Household contacts of a positive COVID-19 person who do not have COVID-19 symptoms themselves, are no longer recommended to test daily for 5 days with a rapid antigen test (RAT). Only household contacts who develop one or more COVID-19 symptoms are recommended to stay at home and RAT test for COVID-19.
You can find out more about this change on the Health New Zealand | Te Whatu Ora website
June 2023
Matariki holiday vaccine ordering – 28 June 2023
There are no deliveries on Friday 14th of July due to Matariki holiday. Providers with Friday deliveries should order extra stock for delivery on Friday 7th of July.
Toolkit to support planning and delivery of Covid-19 immunisations in ARC facilities and disability support services – 28 June 2023
This toolkit has been developed by Te Whatu Ora Southern COVID-19 Vaccination Programme. The toolkit is intended to provide additional information, templates and useful “tips” to support those COVID-19 vaccination providers who deliver COVID-19 vaccinations in age residential care facilities (ARCs) and disability support services (DSS).
Link to toolkit: ARC Facilities Tool Kit 16June2023
If you have any questions concerning the toolkit please contact Judy Walker, Quality Lead COVID-19 Vaccination Programme 027 755 1123; judy.walker@southerndhb.govt.nz
FYI, National Vaccination and Logistics team is reducing staffing – 28 June 2023
The team that supports vaccine logistics at the National level has announced that they are reducing their service capacity. This has resulted in very little ability to support out of schedule deliveries of vaccine.
Recommendations from the national team:
- Order before 3pm, two days before your scheduled vaccine delivery day
- Maintain 3 weeks of vaccine stock where possible
Pēpi enrolment in primary care - survey invitation – 28 June 2023
Healthcare workers are invited to participate in a survey on the challenges they face when enrolling and engaging whānau and their pēpi in primary care. The survey – led by researchers from the University of Auckland and University of Otago – aims to identify primary care actions which will support timely immunisation and protection of tamariki Māori from vaccine-preventable diseases.
Partnering with Hāpai te Hauora, researchers will engage with healthcare workers and whānau whose pēpi were not enrolled within seven weeks of birth to better understand their experiences. Participants will be asked to share their knowledge and opinions about the newborn enrolment process, and what they think is working and what is not.
The survey results will help inform decisions, policies, and practices for the health sector and offer new strategies to address equity gaps, say lead researchers Dr Amber Young (University of Otago) and Dr Samantha Marsh (senior research fellow with the Immunisation Advisory Centre).
Pēpi Māori experience low and late immunisation compared with other children, which exposes them to the risk of vaccine-preventable diseases. Pēpi Māori who are not enrolled with primary healthcare services are less likely to receive their 6-week immunisations on time and are delayed in their protection from diseases.
Who can participate in the survey: Anyone working in a general practice or a community setting looking after a newborn's health and the health of their whānau, either in a clinical or non-clinical role.
Duration of the survey: The survey should take around 10 minutes to complete. Respondents will have the option to go into the draw to win one of five Prezzie Cards.
More information: Please see the Participant Information Sheet, click here, or if you would like to speak with a member of the research team, please get in touch with Dr Amber Young: amber.young@otago.ac.nz
Click here to complete the survey:
https://auckland.au1.qualtrics.com/jfe/form/SV_6Jf5sIZvmnyDn3o
IMAC Webinar: Vaccinating Health Worker (VHW) role – 28 June 2023
Date: Wednesday 5 July 2023
Time: 12pm-1pm
To attend: This is a Teams live event, please follow this link to attend
Te Whatu Ora is hosting a webinar to give an overview on the Vaccinating Health Worker (VHW) role and the new Stage 2 vaccine preparation training now available. The webinar is for any potential or current employers of VHWs, their supervisors or VHWs themselves. The webinar will cover:
- Overview of the VHW role
- Details on the new VHW Stage 2 vaccine preparation training
- Change to the Practical Assessor criteria
- Q&A
Note: Please ensure you have downloaded Microsoft Teams on the device you will be viewing the webinar on, prior to the event.
Referral pathway for COVID-19 vaccination home visits – 28 June 2023
If you know someone who is unable to access a vaccination clinic for a COVID-19 vaccination (due to disability, a mental health or addictions condition, a health condition or other reason), please support them to fill out this form or call 03 476 9977 and we will be in touch to work through a solution.
Book My Vaccine provider alert: Public holidays default to closed – 28 June 2023
From the evening of Wednesday 28 June, the default setting for all appointment schedules on national public holidays will be closed. You will need to apply the closed setting for your regional public holiday if you are closed.
To set your site to closed on regional holidays, if you are a site admin, go to the ‘Holiday Closure’ field in the ‘Information’ section of the ‘Location tab’. Select your region’s name from the drop-down menu.
Please note: If you wish to remain open for all or some national and regional public holidays, please select the ‘Always Open’ option from the ‘Holiday Closure’ drop-down menu and use overrides to manage your site closures.
Book My Vaccine provider alert: Notifications, changing status, and notes within jobs – 28 June 2023
From the evening of Wednesday 28 June, see updates:
Notifications: A new ‘Notifications’ section will be visible on your dashboard below the ‘Today’s Remaining Appointments’ section. The aim is to provide a digital noticeboard for useful information that is easy to access for all users. These notifications will include information about system updates, training resources, when new vaccine types are available, and other useful information.
The notification ‘bell’ icon on the top right of your dashboard will show a red circle when new notifications are available.
Changing the Booking Status of a Job: You will be able to change the booking status of a job directly in the job. To do this you can:
- Search for the consumer and select the job by clicking on the job name.
- Under the ‘Information’ section, select the pencil beside the ‘Booking Status’ field.
- Select the correct booking status from the drop-down menu.
Changing the Booking Status of Multiple Jobs: From the bookings dashboard when you select multiple jobs and click ‘Change Status’, a new table will display the jobs that you have selected to confirm your selection. You can then proceed to change the booking status.
Notes and Comments within Jobs: A new ‘Notes and Comments’ field has been added to jobs. This field can be found directly in the job under the ‘Type of Booking’ section.
By Pacific for Pacific – 21 June 2023
In collaboration with Pacific Public Health and Pacific Health, phase two of the by Pacific for Pacific flu and COVID-19 vaccine campaigns were launched this week to encourage Pacific people across the country to get a flu and COVID-19 vaccination to protect their families and their communities.
‘Shoo the flu’ campaign uses light humour to engage the target audience and deliver an empowering clear message for Pacific families to get rid of the flu by getting the flu jab. A series of video reels have been released on social media platforms, including Facebook, Instagram, Tik Tok and YouTube. This is being supported with advertising across a variety of settings, such as malls, community and sports venues, bus stops and train stations and healthcare clinics. Advertising on bus backs and within buses and trains will also be added from next week.
Informed by recent research and ongoing community feedback, the Get boosted covid campaign uses avatars to reflect the various groups that are eligible for an extra booster. A series of printed and digital posters for each of the avatar groups have been developed and are available as social tiles. A 30-second vox pop video of Pacific people sharing their ‘why’ for getting their COVID-19 jab has also been shared on social media.
Radio ads and radio talanoa for both flu and COVID-19 vaccines have been released in the nine Pacific languages with the help of Pacific clinicians and community leaders answering community Q and A’s each week.
Printed A5 flyers with key information about flu and COVID-19 vaccines will be available in English and also in the nine Pacific languages. Pacific provider and community led vaccination events are being supported to help inform and mobilise Pacific communities to get flu and COVID-19 vaccinations.
Dropbox links to access available resources
- Shoo the flu campaign folder (videos, social tiles, poster) Dropbox – Pacific Shoo the flu campaign
- Get Boosted campaign folder (videos, social tiles, poster) Dropbox – Pacific Get Boosted campaign
From 19 June, you can go to Bluestar to order printed resources for the flu and COVID-19 vaccine collateral (A3 posters and A5 flyers). It is free of charge to order until stocks last.
Boost yourself COVID-19 booster campaign – 21 June 2023
To further support the COVID-19 booster campaign launched in mid-May, targeted digital advertising and social media has recently kicked off.
A series of adverts running on Facebook and Instagram stories and online animated banners reinforce the campaign theme of ‘Boost yourself, Boost your family/whānau’. These adverts directly encourage people to get a booster for stronger protection against COVID-19 and promote the message of ‘boost your immunity’.
The campaign focuses on people incorporating getting a booster as part of their everyday lives. Over the next week a video will also be promoted through various on demand channels, eg, TVNZ on demand, Three Now and YouTube so look out for this.
Update for Covid-19 booster messaging to PMS – 21 June 2023
An update to notifications for all COVID-19 Pfizer vaccinations is planned for Wednesday evening on 21 June 2023. The update will mean that all general practices using a MedTech practice management system (PMS) will be sent a notification whenever an enrolled patient receives a COVID-19 booster for the Pfizer bivalent vaccine (CVX300).
From Thursday 22 June, those practices who use MedTech as a PMS will also receive notifications for enrolled patients who previously received a COVID-19 Pfizer booster vaccination. The backlog of notifications will be sent in batches, starting on the evening of Wednesday 21 June. The backlog notifications will be sent each evening from Monday to Thursday until all notifications are completed.
Notifications for COVID-19 Pfizer booster vaccinations for general practices who do not use MedTech as a PMS started on Friday 10 June.
If you have questions or require support, please email help@imms.min.health.nz.
Ops guidelines update – V56.0 now available on the website – 21 June 2023
The latest version of the COVID-19: Vaccine operating and planning guidelines V56 can be found here: COVID-19: Vaccine operating and planning guidelines | Ministry of Health NZ
Updated Covid-19 vaccine resources – 14 June 2023
There are updated versions of Covid-19 Vaccine resources:
The Goodfellow Unit - Immunisations in the clinical context webinar – 14 June 2023
The Goodfellow Unit will be hosting a Webinar 27th June: An update on increasing immunisation rates in children, with an equity focus, tips and tricks and "busting" common myths of vaccinating in the community. Register here
Vaccine Logistics Updates – 14 June 2023
- Bivalent vaccine stock being delivered currently is short dated; please make sure to use the earliest expiry date first. Make sure to dispose of expired vaccine at close of business on the day of expiry. There have been incidents nationally of expired vaccine being used to vaccinate consumers.
- From July 1, there will be a change in the NZ Post delivery process with the service now provided by NZ Post Pace Express network. The courier will no longer observe the checking process. There is no change to provider delivery days.
- MoH Logistics is asking all providers to maintain 3 weeks stock on hand of Covid vaccine, as with the new delivery process, they want to eliminate out of schedule orders. Maintaining sufficient stock on hand is especially important during the Southern winter months with adverse weather events possibly affecting deliveries.
- If you have any questions or comments, please contact Romilly on (03) 4769915 or 0272115565
Aotearoa New Zealand Immunisation Conference 2023 and pre-conference workshop – 14 June 2023
The wait is finally over! IMAC is excited to share details about the Aotearoa New Zealand Immunisation Conference 2023 and pre-conference Workshop, to be held in Auckland from 15-17 November 2023.
Come and join your colleagues and peers and increase your understanding of vaccine-preventable disease control and immunisation delivery services in Aotearoa. We have confirmed a wide variety of interesting and knowledgeable international and domestic plenary presenters.
Registrations will open in mid-June.
The Conference will be held at the University of Auckland Business School's Sir Owen G Glenn Building, 12 Grafton Rd, Auckland CBD.
Please consider being a Conference presenter yourself and sharing your research, or experiences and practices. There is more information on the Conference webpage, including a link to the Abstract Submissions Portal which is open and ready for your submission.
Please visit immune.org.nz/2023-immunisation-conference for more details.
Sector Stakeholder Hui – 14 June 2023
Thank you to everyone who joined the hui last week. We had a fantastic presentation from the Bay of Plenty team, who shared their approach for Aotearoa Immunisation Week. Additionally, the National Immunisation Programme shared updates about the Aotearoa Immunisation Register (AIR), Book My Vaccine, in addition to operations and communications.
You can find Stakeholder sector hui resources, including slides, video links and a recording of the hui, here: Dropbox – Stakeholder hui 7 June 2023
Aotearoa Immunisation Week update – 14 June 2023
A massive thank you to everyone within the Districts, Providers, Primary Care and Community Health services across the motu who supported Aotearoa Immunisation Week from 29 May to 4 June.
In total, there were 2,709 immunisation activities across the week, which include standing clinics with availability through Book My Vaccine, outreach services, promoted practices and community immunisation locations. Additionally, across the motu there were 168 amplified events, providing access and visibility to the importance and ease of getting immunised across our communities.
Video resources
A series of videos were posted on Te Whatu Ora social media channels throughout the week, highlighting some of the vaccination opportunities available. We’d like to give a special shout out to the providers who accommodated this filming amongst an already busy day!
You are welcome to use any of the videos for your own social media channels, which are available on Dropbox.
Aotearoa Immunisation Week update – 7 June 2023
We acknowledge our vaccination providers for your collective effort and support as you continue to help protect our most vulnerable across the motu.
Last week there have been over a thousand immunisation opportunities where people have been taking advantage of extended hours at nearby clinics, pharmacies, GPs and hauora providers. This is in addition to outreach services to rural communities as well as pop-up clinics at farmers markets and rugby games. Thank you.
A video from River Ridge Birthing Unit in Hamilton was shared on Facebook. There’s also a short promotion of the week and more to be uploaded over the coming days.
You can access and share these videos with your communities: Aotearoa Immunisation Week – video for social media
Update on COVID-19 messaging to PMS systems – 7 June 2023
Notifications for all COVID-19 vaccinations were enabled on Thursday evening, 8 June 2023. This includes notifications for general practices whenever an enrolled patient receives a COVID-19 booster.
Notifications will only be activated for general practices who use any patient management system (PMS) other than MedTech.
Notifications for those general practices who use any MedTech PMS will be activated before the end of June.
Those practices who do not use MedTech as a PMS will also receive notifications for enrolled patients who previously received a COVID-19 booster vaccination. The backlog of notifications will be sent in batches, starting on the evening of Thursday 8 June and continuing again from Monday evening 12 June until Thursday evening 15 June.
May 2023
Updated National Immunisation Programme Operating Guidelines Covid 19 Vaccines Version 55.0 – 31 May 2023
Version 55.0 of the National Immunisation Programme Operating Guidelines has been released and can be accessed through the link below.
Please refer to page 3 Section A for a summary of the changes.
Cold Chain Breach Risk Mitigation – 31 May 2023
Cold chain breaches (CBC)
CCB can occur for a number of reasons, however, there are some things that you can do to help reduce the risk:
1. Reduce the risk that someone accidentally unplugs your fridge. Ensure the plug is inaccessible and/or is clearly labelled ‘Do not disconnect this vaccine fridge’.
2. Do not hold the door open for too long. Make sure you know what you are looking for when you open the door. If you have a solid door on your fridge, have a map on the front to show where each vaccine is situated.
3. If your fridge is in a patient area, make sure it is secure. Lock the fridge so only your staff can open it.
4. Check the fridge door seals to ensure they close tightly.
5. Make sure your fridge has its routine annual service.
On-site immunisation chilly bins
• Must use either a minimum/maximum thermometer or a data logger with an external display, remote probe, i.e., attached to the data logger by a cable, and visible/audible alarm.
• Have the temperature monitored throughout the time vaccines are stored in the chilly bin.
The provider must:
• Document the minimum, maximum, and current temperature every 20–30 minutes after putting the vaccines in the chilly bin, and
• Review the documented temperatures and take action to prevent a cold chain event from occurring if required.
Off-site immunisation chilly bins
• Need to be solid walled and have a clip to hold the lid in place.
• Must use a data logger with an external display, remote probe, i.e., attached to the data logger by a cable, and visible/audible alarm.
• Have the temperature monitored throughout the time vaccines are stored in the chilly bin.
The provider must:
• Document the minimum, maximum, and current temperature every 20–30 minutes after putting the vaccines in the chilly bin.
The data logger must:
• Record the temperature every 5 minutes
• Download, review, and save the data after returning to the clinic
• Take action if required.
If you aren’t familiar with the steps involved in preparing your chilly bin and the documenting process required please take the time to watch this video and review the cold chain resources available on our website cold chain page.
Vaccine Storage and Transportation – 31 May 2023
Vaccines at the provider level must be stored between +2°C and +8°C at all times. Temperature-monitored chilly bins must be used to store vaccines when they are not in the provider’s pharmaceutical refrigerator.
Reminders:
- All providers must be undertaking the minimum monitoring required, which includes:
- A daily min/max reading – which must be documented and follow-up action taken if outside the +2°C and +8°C range
- A weekly check of the continuous monitoring monitor (datalogger or cloud-based systems)
- Both of these checks must be done and documented regardless of the type of system the provider uses. Using the Annual Cold Chain Management Record is a great way to keep your fridge cold chain information in one place.
- It is also really important that you are able to identify what day the vaccines in your fridge arrived at your practice (in case you should have a cold chain breach or if there is a regional or national recall of vaccines). Do this as part of the receipting and unpacking process when vaccines arrive at your clinic by placing a sticker on the vaccine box with the arrival date or writing the arrival date on the vaccine box.
- Don’t forget to update your clinic's Cold Chain Management Policy annually. This will help ensure you have the appropriate processes and systems in place and is an opportunity to update any new staff.
Flu and Covid-19 vaccination television commercials – 31 May 2023
Two television commercials have been created in partnership between Te Aka Whai Ora and Te Whatu Ora to encourage Flu and COVID-19 vaccinations. The intention of the ads is to show Māori in recognisable environments and lived experiences while portraying a degree of humour and then finish with the immunisation message. We've chosen this format so that immunisation is not overbearing in the individual’s mind but the ad is memorable and gives whānau a subtle reminder to go and get their immunisation.
The first of the commercials aired last night and is intended to reach Māori audiences in key programming across TVNZ, Discovery, Sky and Māori TV. It will be supported by online video-on-demand to efficiently extend reach amongst target audience. It will include a modest radio layer to extend the campaign across Iwi stations, Mai and Flava will bolster the campaign launch.
There will be some targeted digital outdoor small format media, will skew towards Māori population, across the four districts, using placements which will not be subject to vandalism.
This is one component of our focused COVID-19 and Flu campaign. A range of other assets for providers are being developed to support their (and your) efforts, which we hope to have available over the coming days. Additional assets for our Pasifika campaign will be available next week.
National Immunisation programme - stakeholder hui – 24 May 2023
Date: Wednesday 7 June 2023
Time: 1.30 to 2.30pm
Link: Click here to join the Teams meeting
We warmly invite you to attend our next stakeholder sector hui which is being held from 1.30 to 2.30pm, Wednesday, 7 June 2023.
The hui is designed to keep our vaccination and immunisation teams up to date with plans and activities undertaken by the National Immunisation Programme. In this hui, we will give you a wrap up summary of the events around the recent Aotearoa Immunisation Week, as well as programme updates from the team on workforce, clinical, operations, equity, campaign, and AIR.
We welcome your questions and feedback. You'll have the opportunity to post questions ahead of the hui. Please email them to sandy.thambiah@health.govt.nz by 6 June 2023.
We hope you can join us, but if you can’t we will share a link to the recording in the following pānui.
Please share this invitation with anyone who may be interested in attending.
Bivalent COVID-19 vaccine ordering – 24 May 2023
MoH Logistics have an abundance of 2 and 5 packs of bivalent that are short dated.
Effective Immediately, 10 packs of 15/15 mcg Bivalent vaccine have been temporarily withdrawn leaving only 2 and 5 packs available to order from the Inventory portal.
Once sufficient stock has been distributed, 10 packs will be available to order again.
King's birthday and Matariki public holidays (Book My Vaccine)– 24 May 2023
An update has been made to all sites to close appointments on the King’s Birthday (Monday 5 June) and Matariki (Friday 14 July) public holidays.
If you would like to offer vaccination appointments on either/both of these public holidays, please email help@imms.min.health.nz to request that your appointment schedule(s)
Extension of Book My Vaccine closing date for appointments – 24 May 2023
The closing date for appointments on BMV can now be extended to 28 February 2024.
To assist you, all active sites with a closing date of 30 June 2023 will automatically be extended to 28 February 2024. This change is planned for the evening of 7 June.
If you do not want the closing date for your location(s) to be automatically extended to 28 February 2024, before 6 June you must either:
- Change your closing date from 30 June 2023 to your required closing date. If you are a site admin user, you can change the date in the ‘Closing Date’ field for your site. You will find the ‘Closing Date’ field within the ‘Information’ section of the ‘Details’ sub-section of the ‘Location’. If you have more than one appointment schedule, you must update the closing date on each location.
- Email help@imms.min.health.nz and request support to update your closing date or to opt-out of the extension.
- If your site is no longer active or available to accept appointments, please adjust the status to pending or email a request to the help desk to decommission your site.
Please be sure to generate any required overrides for time that differs from your default calendar – for example, the weeks between and around Christmas and New Years Day.
Aotearoa Immunisation Week Update (29 May to 4 June) – 24 May 2023
Aotearoa Immunisation Week features locally-led events across the motu that celebrates the importance of immunisations and provides a variety of vaccination opportunities to protect whānau as they prepare for winter.
The aim is to drive vaccine uptake ahead of winter with a call-to-action for whānau to go to their local health provider events. Aotearoa Immunisation Week will include over 35 events, plus hundreds of immunisation opportunities with clinics, pharmacies and GPs across the motu.
Promotions on national radio will create awareness of Aotearoa Immunisation Week, in addition to a media statement to announce the week to the media and general public. The vaccination opportunities and events will be supported nationally by the COVID-19, flu, and childhood immunisation advertising campaigns currently in market across Aotearoa.
A link to events will be available next week on Te Whatu Ora immunise.health.nz website which will also link to Bookmyvaccine.nz for vaccination appointments at existing clinics, pharmacies and GP offices.
Key messages to support Aotearoa Immunisation Week
- Being immunised is one of the best things we can do to protect ourselves, our whānau and our communities from a range of preventable diseases that can cause serious illness and even death.
- The risk of severe illness this winter is high, so we’re encouraging everyone across the motu to be protected.
- Aotearoa Immunisation Week (29 May to 4 June 2023) is a series of locally-led events that celebrates the importance of immunisations and provides a variety of vaccinations to protect whānau as they prepare for winter.
- Aotearoa Immunisation Week will include over 35 events, including hundreds of immunisation opportunities with clinics, pharmacies and GPs throughout the motu.
- The goal of Aotearoa Immunisation Week is for more children, adults, whānau – and communities – to be protected from vaccine-preventable diseases, allowing them to live happier, healthier lives.
- For more information go to www.immunise.health.nz
- As always, the existing suite of collateral to support your vaccination opportunities, which complement the national in-market advertising campaigns for COVID-19, flu, and childhood immunisations, are available on Dropbox now and for ordering on Bluestar.
Holiday Planning: Kings Birthday 5th June – 23 May 2023
Another reminder that there will be no vaccine deliveries on Monday and Tuesday, the 5th and 6th of June. If your delivery day is affected and you have not ordered sufficient stock for two weeks, please contact Romilly ASAP on (03) 4769915 or romilly.smith@southerndhb.govt.nz
Aotearoa Immunisation Week – 18 May 2023
A host of vaccination events taking place around the motu in the lead up to winter are being brought together as part of Aotearoa Immunisation Week from 29 May to 4 June.This is an opportunity for health providers to promote areas they are focusing on in their communities, whether it’s childhood immunisations, influenza, measles, whooping cough or COVID-19 vaccinations.
The aim is to increase our protection against illness ahead of winter with a call-to-action for local people to go to their local health provider events.
The weeklong series of locally organised events will be supported by an already extensive COVID-19, flu, and childhood Immunisation advertising campaigns currently taking place across the motu.
Social media tiles will be available for event organisers and health districts to customise to promote your local vaccination events. An increased stock of posters will also be ready for you to order through Bluestar. Please order these as soon as possible to ensure they reach you in time.
Our regional leads are already in touch to see what health sector events are currently planned and will provide logistical support as required.
More details to follow.
We are supporting Aotearoa Immunisation Week with:
- A Te Whatu Ora media statement to announce the week – to be published week of 22 May 2023
- Proactive media opportunities on national news networks
- Social media content on Te Whatu Ora channels
- Radio ads, prompting the public to visit immunise.health.nz to find out where an event is in their region
- An editable poster, which will be available on Dropbox and Bluestar this week, for providers to either order and fill in event details manually, or download, fill in digitally with event details and print on-site.
- A social media tile, to complement the look and feel of the poster. This will also be added to Dropbox this week and I will circle back to let everyone know when it’s there.
- Hosting an events page on the new www.immunise.health.nz website with details about Aotearoa Immunisation Week, and linking to regional websites where event information is listed. We will link to Book My Vaccine and Health Point for BAU vaccination clinics and appointments (e.g., pharmacies, GP offices, etc)
- Collateral: We also have a full suite of existing collateral to support your vaccination opportunities, which complement our in-market advertising campaigns for COVID-19, Flu, Childhood Immunisations, etc. These are available on Dropbox now and for ordering on Bluestar. We are printing additional resources this week so they are on-hand and ready to ship ahead of Aotearoa Immunisation Week. Please note that to ensure you receive your print order ahead of 29 May, you must order this collateral by Friday, 19 May. You may, of course, order after this date if you simply need to top up some collateral for your event later in the week. Providers will also be advised of this deadline.
- On-going in-market advertising campaigns for Flu, COVID-19 and Childhood Immunisations.
We encourage providers to display this poster: Aotearoa Immunisation Week 2023 A3 Poster
Off-site vaccination set up, including providing services into Age Residential Care Facilities – 18 May 2023
When delivering outreach/mobile/off-site vaccination services it is important to ensure all of the appropriate requirements are met including the preparation, transporting and storing of vaccine.
Please refer to the National Immunisation Programme Operating Guidelines COVID-19 section 8.7. Transportation of diluted or drawn-up vaccine, which outlines the bulk preparation of pre-drawn-up syringes for transporting to another location is not permitted. This includes providing COVID-19 vaccination services in venues such as age residential care facilities.
New pregnancy immunisation collateral – 18 May 2023
There is new pregnancy immunisation collateral in the Dropbox: Dropbox - Pregnancy Immunisation
- NIP 8704 What you need to know about immunisation during pregnancy
- NIP 8706 After your immunisations during pregnancy
- NIP 8705 Immunisation during pregnancy consent form
Vaccine types included on these resources include Boostrix, Influenza, and COVID-19.
These are currently orderable via the Bluestar portal.
Reminder on checking packing slips on receipt – 17 May 2023
There has been an increase in the incidence of vaccine packs being left in shipping boxes after delivery and returned to warehouses.
ALWAYS check the packing slip for:
- Correct ship to name and address before opening the shipping box.
- All items received, including batch details to ensure stock matches.
- Conduct visual checks of outer packaging for damage and/or leakage. If there is no damage store directly in the fridge. If outer packaging is damaged inspect vials.
- Contact NIP Logistics Customer Service (covid-19.logistics@health.govt.nz) if stock received does not match with packing slip or damaged vials.
Novavax expiry update – 17 May 2023
All current stock of Novavax expires on 31 May 2023. If you are a Novavax provider, please order prior to this date to replace the May 2023 expiry stock.
Please make sure to remove and destroy expired stock and waste in the CIR inventory portal.
The new expiry date of Novavax will be November 2023.
Covid-19 Vaccinator Working under Supervision role authorisation – 17 May 2023
An important reminder that from 1 June 2023, the COVID-19 Vaccinator Working Under Supervision (CVWUS) roles will no longer be able to vaccinate. We strongly encourage all CVWUS to transition to become a Vaccinating Health Worker (VHW) by 1 June 2023, so they can continue to be authorised to vaccinate.
The VHW role builds on the success of CVWUS and extends their scope by being able to administer more types of vaccines to a range of ages.
To learn more about the VHW role visit the Te Whatu Ora website.
To access training now to begin the VHW learning pathway, head to the IMAC website for the Vaccinating Health Worker Stage 1 Online course.
Updates to the eligibility for an additional booster – 17 May 2023
The eligibility criteria for additional COVID-19 boosters has been updated to clarify that all young people aged 12 to 15 who are at higher risk of severe illness from COVID-19, may receive an additional booster, with a prescription.
To reflect the eligibility extension on 1 May 2023 to include pregnant people aged 16 and over, the guidance has also been clarified to include this age group in the ‘recommended’ category.
The additional COVID-19 booster is available for:
- people aged 30 and over
- people at higher risk of severe illness from COVID-19 aged 16 to 29
- pregnant people aged 16 to 29 years
- young people aged 12 to 15 who have health conditions that put them at higher risk of severe illness from COVID-19, with a prescription.
Change to CIR early vaccine warning – 17 May 2023
Changes to the COVID-19 Immunisation Register (CIR) that have been made on Monday 15 May 2023 to better reflect the advice provided to all sites.
When recording a consumer’s first booster, the early vaccine warning message has been changed to display if the gap from the last primary dose is less than 150 days (approximately 5 months). When recording any other booster, the early vaccine warning message has been changed to display if the gap from the last booster dose is less than 118 days (approximately 4 months).
Updated "After the COVID-19 Vaccination" fact sheet – 17 May 2023
The “After the COVID-19 Vaccination” fact sheet has been updated to include information about Post Vaccine Symptom Check. The current one (March 2023) is still able to be used, however when you next place your order with Bluestar, this new information will be included. The fact sheet is available here: Dropbox – COVID-19 After the vaccination fact sheet.
Please note that if you have any version of the fact sheet which is older than March 2023, it is out of date. To ensure you are using the latest version and the most up to date content, please check the ID and date at the bottom of the page.
New childhood immunisation resources – 10 May 2023
By Māori for Māori
In collaboration with Te Aka Whai Ora, new Māori specific childhood immunisation resources promoting the benefits of immunisation are now available on Dropbox - Childhood immunisation - Simplify your life.
There are three videos that you are encouraged to share widely:
- The first video is with Dr Rawiri McKree Jansen who takes the sting out of immunisation by explaining how they keep us safe. He also provides the ingoa Māori for immunisation which is ‘he rongoā ārai mate’ which translates to a medicine that prevents illness.
- The second video provides some new kupu for immunisations like polio, rotavirus and measles.
- The third video is reo rua and a kōhanga reo whānau explaining the importance of looking after tamariki and mokopuna and why they chose to get their pepi immunised.
Four social media tiles are also available in poster print format and digital screen displays to promote immunisation. Each poster has a different message, including: Get your immunisations up to date, Keeping your mokopuna safe and Protecting our tamariki for life.
Posters can also be ordered via Bluestar.
By Pacific for Pacific
In partnership with Pacific Public Health, Pacific-specific childhood immunisation resources have been developed to engage Pacific parents and families, to help prioritise immunisations for their children. The resources include two short videos and posters of Pacific parents prioritising their children’s immunisations while balancing their family responsibilities. An additional poster has been developed with professional rugby player, Dhys Faleafaga, with her twin boys.
The videos, posters and social tiles are available on Dropbox and the posters can be printed and ordered via blue star portal.
Posters will be available in the Bluestar portal and can be ordered. Once on the portal, orders will go on backorder until the stock is printed.
Disability access resources – 10 May 2023
We are pleased to let you know that the following accessibility resources are now on Dropbox as well as on the IMAC influenza website www.influenza.org.nz for use at vaccinating sites and pop-ups. These resources have been created by the CCHV District who have kindly shared with you all. These are also helpful for other vaccinations such as COVID-19, as well as non-vaccinating operations, so we encourage you to share these widely.
- Disability Guidance for flu vaccination sites – a guidance document that outlines what a site needs to know and do to ensure vaccinations are accessible.
- Providing Equitable Access poster for staff – please place somewhere visible for staff to refer to quickly
- Alternative Pathway Sign – please use this to identify a specific location in your facility for people to go to if they need extra time, space or assistance.
- Hello I’m Deaf and Hello I’m Hard of Hearing Communication Cards – please make these visible and available to people entering the facility so that they can choose one if they need to, and offer them to anyone who has indicated they are Deaf or Hard of Hearing
- Name and pronoun cards – please make these visible and available to people entering the facility so that they can use one if they need to
- Communication Cards Posters – please place somewhere visible to alert people that the communication cards are available.
Importance of using the “NOTES” function in CIR – 10 May 2023
The functionality of the CIR Adverse Events section in the CIR has changed.
This means when an Adverse Event is entered into the CIR by the vaccinator CARM is automatically notified and this remains red flagged as an Adverse Event in the CIR.
However, no details of the Adverse Event can be accessed by future CIR users – for example, if the Adverse Event was due to a client’s post vaccination allergic response or an early vaccination was given in error, this event information is not visible in the CIR.
For this reason, it is very important the “NOTES” section of the CIR is completed to include information relating to every Adverse Event including vaccine errors to ensure a comprehensive record of actions is visible.
Extension of Additional boosters from 1st May – 10 May 2023
Additional boosters of COVID-19 vaccine are now available for those from 16 years of age, for those who match the funded influenza vaccine criteria set by Pharmac, (those criteria that relate to health conditions, rather than age or employment) including the mental health criteria.
Pregnancy vaccines are now recommended for those aged 16 years and over with additional health risks and are available to those who are otherwise healthy. Nuvaxovid is not the recommended vaccine for use in pregnancy. If used it will require documented informed consent and a prescription.
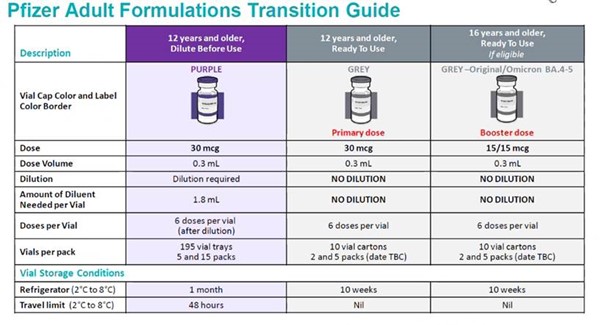
Additional doses for those aged 12-15 years This age range is not part of the programme but additional doses may be recommended for those at risk of severe disease refer to the Starship website. A prescription and written consent is required. The Comirnaty Bivalent 15/15 grey cap is the vaccine of choice and the data sheet allows the administration of boosters from 12 years of age.
IMAC is currently updating the resources on its website and they will be available soon.
COVID-19 vaccine messaging from CIR to General Practice PMS – 10 May 2023
There is currently no messaging of COVID-19 vaccinations from CIR to PMS systems.
The change to the Pfizer bivalent booster vaccine has caused an issue with GP notifications/messaging. All PMS vendors needed to make a change to their systems to accept messages for this new vaccine type.
We are waiting for one vendor to make the change and can then reactivate the messaging for this vaccine from CIR.
The National Programme is hoping that the PMS vendor will be able to provide a timeframe for the completion of this change next week.
Te Whatu Ora - Southern retires clinic finder page – 3 May 2023
Many of you will be aware that Te Whatu Ora Southern has maintained a "Clinic Finder" page on its website to assist people in finding their nearest vaccine clinic.
From Monday 8 May, we will be retiring this page and instead directing users to visit Healthpoint to find their most convenient vaccination location. Healthpoint is already used as a reference for Healthline to support people in accessing clinics and is the channel used by national campaigns.
For those of you still using the "Find a COVID-19 vaccination clinic near you" business cards, the link will be redirected to ensure customers can still find what they are looking for.
To ensure your clinic's visibility and quality customer service, it is important that you keep your Healthpoint profile up-to-date. This includes your operating hours, services provided and preferred booking methods. If you require any help with your Healthpoint page, please contact helpdesk@healthpoint.co.nz.
Book My Vaccine schedules – 3 May 2023
If you have not already done so, please check and update your forward booking capacity in Book My Vaccine to ensure your clinic is visible to service users and we continue to offer convenient vaccination options for our communities.
If you need further help support is available:
Drop-in Coaching: If you have questions and would like coaching with one of our team live, please join a drop-in Teams session. The team is available between 1pm and 1.45pm each weekday.
Email and Phone Support: The team is available to answer questions and provide support. Please email help@imms.min.health.nz or call 0800 223 987. These channels are currently monitored:
- 8am - 5pm, Monday to Friday (from 9.30am on Wednesdays)
- 9am - 2pm, Saturday and Sunday
If you are contacting via email (recommended), please provide a detailed description of the issue, your full name and your mobile number so we can contact you with a resolution
More information
Useful information about Book My Vaccine can be found on our website. This details the key changes for providers using Book My Vaccine.
Second checker competency checklist – 3 May 2023
IMAC has created a second checker competency checklist (published 2 May 2023).
Once a person has completed the IMAC second checker course, they will receive this competency checklist which will need to be shared with their Employer to show the competency has been completed.
Recording an overseas vaccination in CIR – 3 May 2023
Recording an overseas vaccination in CIR - Quick Step Guide
Important: Where a consumer has received one or more COVID vaccinations overseas, all these overseas vaccinations must be recorded against the consumer’s Standard Plan Immunisation Case, including any booster doses of a COVID vaccine they have received overseas.
This means, where a consumer has completed their expected standard course e.g. the consumer has completed 1 dose of Janssen or two doses of Pfizer or AstraZeneca and has received one or more doses above the expected standard course overseas, these vaccinations must be recorded using the ‘Add Vaccination’ functionality on the existing Standard Plan Immunisation Case, not under a Booster Plan.
You can also find this guide on CIR here: Recording an Overseas Vaccination in the CIR - Quick Step Guide (site.com)
Or here: https://ncts.my.salesforce.com/sfc/p/4a0000008aXT/a/4a0000002E2h/C8p6NVjg0C8Gj3m.aEWXJvxiNy9soOglB.Uuh0gTDsc
If you need assistance, please contact help@imms.min.health.nz or 0800 223 987.
Important information for providers vaccinating ARC Facilities – 3 May 2023
An important reminder for all Covid-19 Vaccination providers working to provide vaccinations in age residential care facilities and disability support services.
- Please ensure age residential care facilities and disability support services always have the most up-to-date COVID immunisation information including eligibility criteria, and understand how this applies to individual residents/clients
- Please ensure if providers are obtaining written Informed Consent for COVID-19 vaccinations, from designated personal representatives, for those who are unable to consent themselves that the consent is genuinely “informed” and personal representatives receive full and appropriate information to inform their decision-making. There should always be the option for the personal representative to have a direct conversation with an authorised vaccinator if they have any questions or concerns.
- Please ensure if providers are obtaining written Informed Consent for COVID-19 vaccinations, from designated personal representatives, for those who are unable to consent themselves – in advance - that only those residents/clients who will meet the COVID-19 vaccination eligibility criteria on the scheduled vaccination date (site visit) receive requests for written Informed Consent
- If there are vulnerable residents/clients who may require a COVID-19 booster earlier than the recommended programme eligibility—this will require a written Informed Consent and discussion with an authorised vaccinator. Note depending on the resident's/client's circumstances a prescription may also be required.
COVID-19 vaccine stock expiry and updates – 2 May 2023
Adult Pfizer 30mcg, Paediatric Pfizer and Infant Pfizer: Due to slow movement of stock, 10 packs of Adult Pfizer 30mcg, Paediatric Pfizer and Infant Pfizer have now been withdrawn from the pickable stock at the warehouse. Please order 2 and 5 packs of these vaccines.
Adult Pfizer 15/15mcg is still available in 10 packs, as well as 5 and 2 packs.
Novavax: Batches expired on 30 April so please check your stock expiry date and, if necessary, urgently waste stock off CIR and destroy it. If you have not ordered and cannot wait for the new batch, replacement stock expiring 31 May is available now for reorder. The new batch will be available later this week with a November expiry.
We appreciate the challenges you are all facing with changes to demand but please try to manage all COVID-19 vaccine stock carefully to ensure you have enough on hand whilst minimising wastage.
Extended COVID-19 booster eligibility – 2 May 2023
On 1 May, eligibility for an additional COVID-19 booster was extended to all pregnant people aged 16 to 29 years, to align with the flu age group criteria set by Pharmac.
Eligibility from 1 May
- The additional COVID-19 booster is available for:
- people aged 30 and over
- people at higher risk of severe illness from COVID-19 aged 16 to 29
- pregnant people aged 16 to 29 years
- severely immunocompromised young people aged 12 to 15.
- Boosters are especially recommended for:
- all people aged 65 years and over
- Māori and Pacific people aged 50 and over
- people living in age or disability residential care facilities
- people aged 30 and over at risk of severe illness if they get COVID-19
- pregnant people with health conditions that put them at higher risk of severe illness from COVID-19
- people with disability with significant or complex health needs
- people with serious mental health conditions
- severely immunocompromised young people aged 12 to 15. Talk to your usual doctor, nurse, or healthcare provider about whether this is recommended and how to get a prescription.
Additional messages
- The bivalent vaccine has replaced the Pfizer COVID-19 vaccine for boosters.
- The Pfizer COVID-19 booster causes the immune system to create antibodies against both the original variant of SARS-CoV-2 and Omicron subvariants.
- The vaccine used for the primary vaccination course is the original Pfizer COVID-19 vaccine. The Novavax vaccine isn’t recommended for pregnant people, please consult with a health professional.
- We encourage everyone who is eligible, to get both their COVID-19 booster and flu vaccination to ensure that they are well-protected ahead of Winter.
Background key messages for additional booster dose
- To get an additional COVID-19 booster you must have had at least your first two COVID-19 vaccinations.
- It’s recommended that you wait at least 6 months if you have had a COVID-19 infection. People at higher risk of serious illness can consider an additional booster from 3 months after COVID-19 illness.
- After a COVID-19 infection your antibody levels will be high, giving you some protection. This gradually decreases over 6 months which is why an additional booster dose isn't recommended until 6 months after infection.
- If you want to have an additional booster earlier than the recommended gap after your last COVID-19 vaccine, you should discuss this with your healthcare provider. You might not need a prescription.
- If you are at higher risk of impact of Flu or COVID-19 illness, please consult with a health professional on the right time for you to get an additional booster dose, it may be earlier than 6 months.
- Staying up to date with the recommended COVID-19 vaccinations will continue to protect you from the risk of serious illness, hospitalisation or death from COVID-19.
- Novavax additional booster doses continue to be available for eligible people aged 18 or older, 6 months after their last vaccination or infection with COVID-19. Prescriptions are not required, however Novavax is not recommended for pregnant people.
- You can book your COVID-19 vaccine or booster at www.BookMyVaccine.nz or by calling the COVID Vaccination Healthline on 0800 28 29 26 (8am to 8pm, 7 days a week).
Additional messages specifically for the health sector
- Healthcare, age care and disability workers who are aged 16-29 are not eligible to receive an additional booster unless it is prescribed.
- Those who wish to have the original Pfizer vaccine (if over 16) or Novavax vaccine (if over 18) as their additional booster dose can choose to do so when presenting for their vaccination. Note that Novavax is not available at every vaccination centre.
- For 16- to 29-year-olds, at-risk groups are eligible rather than recommended to receive an additional dose. However, those in this age group with more severe conditions or multiple comorbidities should particularly consider an additional dose.
- Those with more severe conditions or multiple comorbidities should particularly consider an additional dose. The benefit of vaccination in the reduction of severe COVID should be weighed against the small risk of vaccine-associated myocarditis. The latter risk is higher in males aged up to 30 years.
- The Pfizer booster is the preferred choice during pregnancy due to the lack of safety data for Novavax.
Resources ordering through the Bluestar Portal – 2 May 2023
Bluestar, the organisation responsible for printing and distributing vaccination resources, is currently low on stock of both HP8682 After Your Flu Vaccination and HP8591 After Your Covid Vaccination resources and are unable to fulfil orders for the next couple weeks. If you require assistance, please contact Lachlan.Winter@southerndhb.govt.nz or 034769977.
Reminder: it remains very important to complete a full informed consent process prior to vaccination and to provide clear post-vaccination information for clients to photograph or take away. If you do not have these resources on hand please get in touch. You can also print these resources from the National Immunisation Programme Dropbox.
April 2023
If a person has had a COVID-19 vaccination overseas – 26 April 2023
Those vaccinated overseas with any COVID-19 vaccine and aged 16 years or over are eligible for an additional dose of Comirnaty vaccine provided it is at least six months since their last COVID-19 vaccine dose or since COVID-19 infection (i.e. recovery from acute illness or a positive SARS-COV-2 test), whichever is later.
NIP Operating Guidelines v54 – 26 April 2023
On Friday 21 April, the National Immunisation Programme published version 54 of the Operating Guidelines. You can download the new Operating Guidelines here, or find them in the Key Links section at the top of this page.
A summary of changes is outlined in the opening pages of the document.
Do not transfer vaccine – 26 April 2023
Please DO NOT transfer vaccine without prior arrangement from your Logistics Coordinator (Romilly 034769915).
Please order more vaccine at each delivery so you have ample stock on hand; in emergencies, please call Romilly to arrange an out-of-schedule delivery.
Second checker course now live via the IMAC Learning Portal – 24 April 2023
The Second-Checker course is now available via the IMAC website and registration is free.
- The course is approximately 2 hours long, completed online, includes an assessment, and on completion generates a certificate.
- Access is via IMAC’s new LMS – Second Checker Course (LINK)
Who it is for
This course is designed for non-registered team members such as kaiāwhina, health care assistants, administration staff, pharmacy technicians and assistants to enable them to complete independent specific checks of the vaccine preparation process in settings where there may be limited clinical staff onsite.
The primary aim
The primary aim of the Second Checker role is to reduce the risk of vaccine errors. The authorised vaccinator (a registered health professional) has overall responsibility for checking vaccines before administration to a consumer, however, a team member that completes the Second Checker course can work alongside the registered vaccinator to support accuracy.
Free access to all healthcare professionals
This is an amazing resource for non-clinical staff to get involved in the vaccinating space, and support clinical staff.
However, we encourage authorised vaccinators, and other clinicians to also complete this free course to understand the content, and what this course enables a second checker to do. It is a good refresher for us all.
Authorisation Expiry for COVID-19 Vaccinator Working Under Supervision (CVWUS) – 24 April 2023
The COVID-19 Vaccinator Working Under Supervision (CVWUS) was introduced as a response to the pandemic in June 2021 as a new class of vaccinators in New Zealand, to accelerate the expansion and diversification of the COVID-19 vaccinator workforce.
The Immunisation Advisory Centre (IMAC) introduced the CVWUS education programme, which resulted in a pathway for the non-regulated healthcare workforce to join the immunisation workforce and increased capacity of providers, particularly Māori and Pacific health providers and pharmacies to administer COVID-19 vaccine to their local communities. The programme ended in July 2022 and has been superseded by the Vaccinating Health Worker (VHW) pathway. A total of 517 CVWUS were authorised by Te Whatu Ora and their authorisation to administer COVID-19 vaccine will expire on 1 June 2023.
This means, if you wish to continue to administer COVID-19 vaccines beyond 1 June 2023, you must become an authorised VHW. In August 2022, VHW was introduced as a new class of vaccinators in New Zealand to provide a career progression pathway for authorised CVWUS to upskill and expand practice scope. This included the ability to administer additional vaccines under supervision including influenza, HPV9 and Tdap vaccines. Further upskilling will enable VHWs to administer vaccines to an extended age group and includes administration of the MMR vaccine.
Any CVWUS that does not transition to a VHW by 1 June 2023 will no longer be authorized to vaccinate and must stop vaccinating on this date.
The VHW education is offered by IMAC. To learn more about the education pathway and enrol into the VHW programme, visit the IMAC website.
To learn more about the VHW role and understand the employer responsibilities visit the Te Whatu Ora website.
- VHW Capability matrix
- VHW Information sheet
- VHW Supervisor information sheet
- VHW Employer information sheet
Childhood Immunisation Campaign – 18 April 2023
From mid-April, the National Immunisation Programme will be running an advertising campaign across the motu, promoting childhood immunisations. The campaign is led with two different TV adverts, updating last years “Acts of Aroha” creative with charming scenes of parents and caregivers protecting their tamaraki.
The objectives of the marketing communications for Childhood Immunisation are to:
- Ensure parents and caregivers prioritise immunising their tamariki
- Are aware that immunising their child is the most effective way to protect them from serious diseases
- Understand that their child needs certain immunisations at specific times throughout their life
- Reassure them that if any recommended vaccinations are missed, they can catch up
A suite of communications activities intended to prioritise whānau who have fallen behind their scheduled vaccinations has been developed.
Appearing on mainstream free-to-air channels, Sky TV, and Māori television, as well as TVNZ+ and ThreeNOW, the TV media will run until 4th June. TV will be supported by targeted YouTube and online video placements, as well as screens in medical centres, partner packs, collateral updates, and immunise.health.nz.
Tik Tok video promotion
NIP recently collaborated with a Pacific social media influencer to help promote childhood immunisations messages.
Dhys Faleafaga is a former Black Ferns sevens player, now playing for the Chiefs and has over 250k Tik Tok followers. Dhys, together with her partner Tone Ng Shiu (current All Blacks sevens player), developed a Tik Tok video with their twin babies with the prioritise to immunise message. After one week, the Tik Tok video had over 180k views and over 25k likes. You can watch it here.
www.immunise.health.nz
Website was launched mid-March 2023 that provides a hub for parents with all the information they need to make the best decision to get tamariki immunised and to catch up where appointments have been missed.
The Immunisation Pānui
The Immunisation Pānui contains updates on direct marketing, advertising, etc. and access to the Immunisation Dropbox which contains all collateral:
- If you do not receive this but would like to be on the list, send an email to Sandy Thambiah (Sandy.Thambiah@health.govt.nz)
- Here is the Dropbox link for all immunisation collateral: Dropbox: National Immunisation Programme – immunisation collateralThis is also li nked in the Key Links section at the top of this page.
NIP Operating Guidelines v53 – 17 April 2023
On Monday 17 April, the National Immunisation Programme published version 53 of the Operating Guidelines. You can download the new Operating Guidelines here, or find them in the Key Links section at the top of this page.
A summary of changes is outlined in the opening pages of the document.
Vaccine stock expiries and ordering – 17 April 2023
Novavax
- Please order fresh stock to replace your current April expiry stock, and when receipted, move your April expiry stock to waste.
- Note the current orderable warehouse stock is May expiry.
- This means that once the next receipt of stock (expected in May) is available, you will need to order again in May.
Pfizer
- Please check primary grey cap expiry (first batch coming up to expiry 28/04/2023)
- Two and 5 vial packs are now available.
- Paediatric Pfizer: we have noted a drop in 10 vial pack orders. The logistics team are reviewing whether to continue stocking 10 vial packs and have stopped supply with immediate effect while this review takes place. Please order 2 and 5 vial packs in the immediate period. We will update you on the outcome of the review.
Please only place one order per delivery date and remember to add all consumables/needles/ syringe labels etc. to your vaccine order.
Book My Vaccine resources and reminders – 17 April 2023
*If you are closed on ANZAC Day, 26 April, please remember to switch off BMV bookings for that day*
Thank you for registering for the BMV webinars and, if you were able to attend, for making the webinar series a success with your participation. Please find a video compilation of the last session here: https://mohnz.zoom.us/rec/share/Aa700OVe6M-AqxBhpWW-qJwXobCxx--6CSAxJEVUC-fjJhk4pclQECYFbQPgmQ_3.5WHbcV7EFe5sQP-I?startTime=1681366445000
The session starts at the 18 min mark.
The team will also be reviewing the questions asked in the three sessions and these will be included in the FAQ and future communications.
If you need further help support is available:
Drop-in Coaching: If you have questions and would like coaching with one of our team live, please join a drop-in Teams session. The team is available between 1pm and 1.45pm each weekday.
Email and Phone Support: The team is available to answer questions and provide support. Please email help@imms.min.health.nz or call 0800 223 987. These channels are currently monitored:
- 8am - 5pm, Monday to Friday (from 9.30am on Wednesdays)
- 9am - 2pm, Saturday and Sunday
If you are contacting via email (recommended), please provide a detailed description of the issue, your full name and your mobile number so we can contact you with a resolution
More information
Useful information about Book My Vaccine can be found on our website. This details the key changes for providers using Book My Vaccine.
Updated Immunisation Handbook v22 – 17 April 2023
On 11 April 2023, the Ministry of Health released version 22 of the Immunisation Handbook 2020.
You can find the handbook online here, or download it in full here. You can also find it in the Key Links section at the top of this page.
WEBINAR: World Hand Hygiene Day – 17 April 2023
Date: 27 April 2023
Time: 1pm-2pm
The webinar will be delivered via Zoom. Please email to register: hhnz@hqsc.govt.nz
Good hand hygiene is one of the simplest, most effective ways to prevent the spread of healthcare-associated infections, which makes it a key patient safety priority.
Te Tāhū Hauora Health Quality & Safety Commission (Te Tāhū Hauora) has led Hand Hygiene New Zealand (HHNZ), a national quality improvement programme to improve hand hygiene practice in Te Whatu Ora districts and private surgical hospitals throughout Aotearoa New Zealand. The programme is part of the Te Tāhū Hauora infection prevention and control programme, which aims to reduce the harm and cost of healthcare-associated infections.
HHNZ uses the World Health Organization’s multimodal hand hygiene improvement strategy to drive culture change and establish best hand hygiene practice for every patient, every time.
In this webinar, the audience will hear from three speakers about quality improvement projects that have been implemented successfully as part of a local HHNZ programme.
Please share this event page with your colleagues and networks.
Who should attend?
Anyone who is involved in an HHNZ programme, including:
- hand hygiene leads and coordinators
- gold auditors
- personnel working in quality and patient safety
- infection prevention and control professionals.
Speakers
- Cath Robbins – National Infection Prevention and Control Programme, Southern Cross Healthcare. Cath will share the campaign that the infection prevention and control team recently rolled out across the Southern Cross hospital network. The campaign uses refreshed hand hygiene posters and hand hygiene ambassadors to improve hand hygiene compliance.
- Carmel Hurley-Watts – Waitaha Canterbury hand hygiene coordinator. Carmel will share the work undertaken in Waitaha Canterbury to improve hand hygiene access for patients and the district’s recent improvements with the visibility of their hand hygiene compliance rates.
- Vicki McKenzie – Infection prevention and control nurse, Bidwell Trust Hospital. Vicki will describe how she introduced the concept of ‘Take a moment’ to allow for point-of-care feedback and reflection on missed hand hygiene moments.
Registration
The webinar will be delivered via Zoom. Please email to register: hhnz@hqsc.govt.nz
If you have questions about this event, please email: hhnz@hqsc.govt.nz.
REMINDER: Holiday vaccine deliveries – 12 April 2023
Due to public holidays, there will be no vaccine deliveries:
- Tuesday 25th and Wednesday 26th April
- Monday 5th and Tuesday 6th June
Please ensure if you have a delivery day on any of those days to order sufficient stock the week prior to tide you over.
Is your healthpoint up to date? – 12 April 2023
Healthpoint has developed its back-end functions to allow providers to better indicate the services they offer. This includes tick boxes for most vaccinations, as well as the ability to add any services that are not listed.
It is really important you keep your healthpoint page up to date with the services you offer, your hours and how people can book in or if walk-ins are available. Nationally led campaigns for a number of vaccinations direct people to healthpoint to find a service near them, and it is also used by Healthline to support consumers to access services. It is especially important for the visibility of those providers who are not using Book My Vaccine for flu and COVID-19 vaccinations.
If you need support updating your page, contact helpdesk@healthpoint.co.nz.
Book My Vaccine: Useful tips and reminders – 11 April 2023
Changes
Book My Vaccine has undergone some changes for providers who have used it for COVID-19 or may be new to some users. Here are some useful reminders and tips for using Book My Vaccine;
- Please log into Book My Vaccine regularly to view upcoming appointments. Flu bookings will not show in AIR or CIR. This means that if providers are not checking their Book My Vaccine schedule a consumer may arrive for a booking that the provider is not aware of. Providers need to ensure they log into Book My Vaccine regularly and view the list of daily bookings. Please note that a consumer can make a same day booking or cancellation, so appointments showing as scheduled may change throughout the day.
- Public Holidays. Public Holidays are not automatically blocked out on Book My Vaccine. Site admins are required to ensure that Public Holidays are accounted for in Book My Vaccine by either blocking them out and rebooking consumers or having the ability to deliver vaccinations if the site is open on that particular holiday. Providers need to review all Public Holidays and bookings to ensure they have the correct provisions in place. This includes Easter (April 7-10), Anzac Day (April 25) , Kings Birthday (June 5), Matariki (July 14) as well as any other regional anniversary days.
- Rebooking Clinics. Consumers are not automatically rebooked if a clinic is cancelled or modified. Sites are responsible for contacting consumers to make alternate booking arrangements.
Immunisations Systems Support
Webinar: Join a 30-minute webinar to find out how to use Book My Vaccine to manage consumer bookings and appointment schedules. Register using this link.
- Thurs Apr 13, 2023 06:30 PM
How-to Guides:
• BMV – How to manage bookings using the dashboard
• BMV - How to manage capacity and operational hours
Drop-in Coaching: If you have questions and would like coaching, please join a drop-in Teams session. The team is available between 1pm and 1.45pm each weekday.
Email and Phone Support: The team is available to answer questions and provide support. Please email help@imms.min.health.nz or call 0800 223 987. These channels are currently monitored:
- 8am - 5pm, Monday to Friday (from 9.30am on Wednesdays)
- 9am - 2pm, Saturday and Sunday
If you are contacting via email (recommended), please provide a detailed description of the issue, your full name and your mobile number so we can contact you with a resolution.
More information
Useful information about Book My Vaccine can be found on the Te Whatu Ora website. This details the key changes for providers using Book My Vaccine.
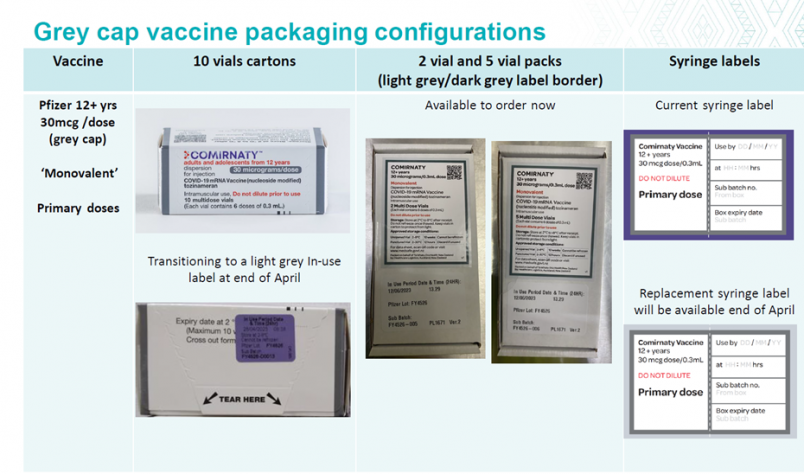
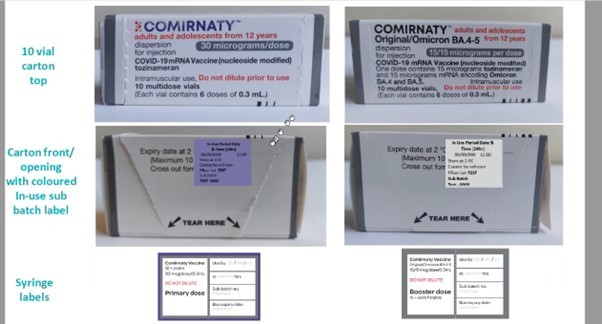
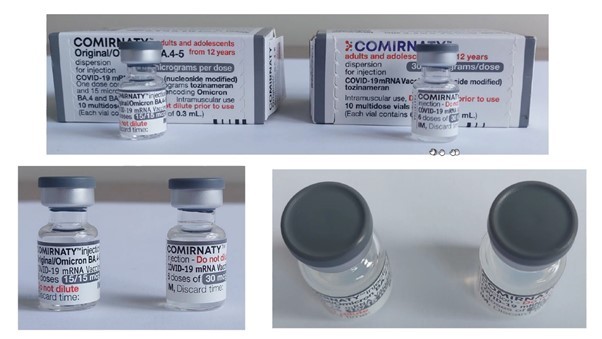
30mcg labels change – 11 April 2023
The light grey/dark grey labels for the 2 vial and 5 vial packs of the 30mcg Pfizer vaccine are available now.
At the end of April:
- there will be a transition in the labelling for 10 vial cartons of the 30mcg Pfizer vaccine
- replacement syringe labels will be available.
Please see the designs below.
NOTE: there will be no change to the current 15/15mcg dose labelling.
IMAC have developed posters to assist with differentiating the grey cap vaccines see here and further tips on safe preparation and storage of COVID-19 vaccines see here.
Release of the Immunisation Taskforce report – 11 April 2023
On 6 April, the NIP released the Immunisation Taskforce Report. The Report provides advice to Te Whatu Ora and Te Aka Whai Ora on how we can best utilise our size and scale to rapidly improve immunisation rates for tamariki and achieve equity across all population groups. The Report includes 54 recommendations, all of which have been accepted by Te Whatu Ora, with 26 already underway.
The media release, which includes a link to the Immunisation Taskforce Report, is published on the Te Whatu Ora Website.
CIR Portal how-to for providers – 11 April 2023
The NIP has produced a handy how-to guide to help you navigate the CIR portal.
Download the guide here.
Updated IMAC resources to support 1 April changes to additional COVID-19 boosters – 11 April 2023
- Comirnaty Grey Cap factsheet v4B (published 1 April 2023)
- Comirnaty Screening Guide v19B (published 1 Aprl 2023)
- Nuvaxovid factsheet v5 (published 28 March 2023)
- Nuvaxovid Screening Guide v9 (published 28 March 2023)
New collateral – 4 April 2023
As we all know there are complex changes as of April 1st for COVID-19 booster eligibility. A great tool has been created by the team as a guide for authorised vaccinators and providers. You can download the additional booster recommendations guide here, or find it in the dropbox linked in the Key links section at the top of this page.
On 31 March, NIP published a Winter Wellness FAQ document for the sector which you can find here.
COVID-19 and Flu collateral is now available in the Dropbox and to order on Bluestar. Combined flu and COVID consent form is intended to be used for COVID-19 additional booster doses and can be used with both the COVID and flu fact sheets to support the informed consent process
Bluestar
- All consent suites (consent forms and fact sheets) are available on Bluestar for pre-order, please allow 1-2 weeks to arrive.
- When ordering stock from the Bluestar portal, please be mindful of how many units you order, each unit contains 25 sheets.
- Please see section 7.3 of the Operating Guidelines for more information on ordering site collateral.
Transfer of vaccine – 4 April 2023
Please DO NOT transfer vaccine without prior arrangement from your Logistics Coordinator (Romilly 034769915).
Please order more vaccine at each delivery so you have ample stock on hand; in emergencies, please call Romilly to arrange an out of schedule delivery.
NIP Operating Guidelines v52 – 4 April 2023
On Tuesday 28 March, the National Immunisation Programme published version 52 of the Operating Guidelines. You can download the new Operating Guidelines here, or find them in the Key Links section at the top of this page.
A summary of changes is outlined in the opening pages of the document.
COVID and myocarditis research – 3 April 2023
A recent article published in The Conversation details research into the risk of myocarditis from COVID vaccines versus risk of heart damage from COVID disease.
You can read the full article here.
Vaccine Checks must be done – 3 April 2023
To reduce the risk of errors, eg using expired vaccine, please ensure that you are following our guidance, which includes checking and double checking dates, age of vacinee, type and dose. It's also good to check your stock levels for re-ordering.
IMAC has resources to help you prevent errors, including the 7 Rights of Vaccine Administration poster.
7 Rights of Vaccine Administration
- RIGHT Person: Check age, identity and obtain informed consent (complete consent form if required).
- RIGHT Vaccine & diluent: Check vaccine name, diluent name, and expiry date and time.
- RIGHT Time: Correct age, appropriate interval, and administer before vaccine or diluent expires.
- RIGHT Dosage: Check the syringe volume is accurate and the right dose for age.
- RIGHT Route, needle length & technique: Correct route of administration eg, intramuscular. Correct needle length for depth of injection. Correct technique eg, 90⁰ angle.
- RIGHT Injection site: Correct site selection eg, deltoid for intramuscular adult injection.
- RIGHT Documentation: Correct details recorded eg, vaccine, diluent, batch, expiry, date and time administered, dose, route and consent details.
March 2023
COVID-19 booster promotion – 27 March 2023
From 1 March, a new Pfizer COVID-19 bivalent vaccine became available to eligible New Zealanders as a booster dose. To ensure those currently eligible for a first or second booster, are made aware of the new booster, a direct communications campaign has been developed that will see email and SMS delivered to approximately 736k individuals.
- The selected audience will be invited to get a free bivalent booster to get a significant pre-winter boost to their protection from severe illness, hospitalisation and death from COVID-19.
- The targeted audience includes all people aged 18+ who have had their 2-dose primary course and are eligible for a first booster, and all those who meet eligibility for a second booster.
- Several exclusions (in addition to standard exclusions) have been applied to refine the targeting criteria such as removing anyone contacted about COVID-19 boosters via any direct channel (email, SMS, outreach call or physical letter sent by Te Whatu Ora) in the last 3 months, persons who have received a communication about MMR vaccination in the past month and people who are scheduled to be contacted and offered a free flu jab in early April.
- The call to action encourages people to visit www.BookMyVaccine.nz or www.healthpoint.co.nz/covid-19-vaccination, call Healthline on 0800 28 29 26 (8am to 8pm, 7 days a week), or contact their GP, pharmacy or healthcare provider.
New Immunisation website – 27 March 2023
We're pleased to let you know the new immunisation website – https://www.immunise.health.nz/ –is now live. This has been a real team effort with many partners, providers and stakeholders involved in getting us to this point.
This new Te Whatu Ora website has been developed to meet a critical gap in the information currently available online to whānau when making decisions about immunisations for their tamariki, rangatahi and themselves. It will also be a key component of a wider programme of focused communications to support our goal to increase childhood immunisation getting underway in April.
Although the site has a child immunisation focus – the content has been written to cover wider whānau immunisations too.
We hope the site feels relevant to all people living in Aotearoa New Zealand - and is warm, friendly, reassuring, and easy to understand.
A new tool we’re particularly excited about is the ability to create a personalised immunisation schedule for a child: https://www.immunise.health.nz/get-a-personalised-immunisation-schedule/
Please take some time to look around and feedback on anything – whether positive or negative. We’re particularly keen to hear what you think might be missing. This is the first phase and we will continue to build on the foundation it presents.
At the moment we’re sharing the site with the sector in order to gather feedback, and in a couple of weeks, we’ll start to actively drive traffic to the site. We look forward to hearing what you think.
IMAC bridging course pathway – 27 March 2023
The IMAC Vaccinator Bridging Course will end on 30 April 2023.
This course is a temporary pathway for eligible and interested provisionally authorised vaccinators to become fully authorised vaccinators.
People who have completed the course but have not completed the other requirements as outlined in the Immunisation Handbook Appendix 4 are encouraged to do this and apply to their local Medical Officer of Health for full vaccinator authorisation.
People who are eligible and interested in this course are encouraged to enrol now. For course dates please view IMAC website and register.
Māori and Pacific immunisation providers who wish to organise an in-person 1-day course for their team before 30 April 2023 should contact the below local IMAC education team.
- Northern - Lisa Box, Northern Regional Immunisation Advisor
- Midlands - Janet Collins, Midlands Senior Immunisation Education Facilitator
- Central – Shelley Kininmonth, Central Regional Immunisation Advisor
- Southern – Sue Rogers, Southern Regional Immunisation Advisor
If you wish to become a fully authorised vaccinator after the closing of the Vaccinator Bridging course, this can be achieved via the Vaccinator Foundation Course pathway. Registered and enrolled nurses and nurse practitioners, and registered pharmacists, are eligible for the Vaccinator Foundation Course.
The Provisional Vaccinator Update Course will end on 16 June 2023.
The authorisation certificates issued by Te Whatu Ora or the Ministry of Health to Provisional Authorised Vaccinators are valid for two years (from issue date). Vaccinator training is also valid for two years from the date it is completed. To maintain authorisation status, vaccinator training must be kept current. If training expires before the authorisation end date, Provisional Authorised Vaccinators must complete the Provisional Vaccinator Update Course to continue to vaccinate.
To access this course, please email Te Whatu Ora, Vaccinator Authorisations Team vaccinatorauthorisations@health.govt.nz to get the package code to access the online course on IMAC’s learning online platform.
If you wish to become a fully authorised vaccinator after the closing of the Provisional Vaccinator Update Course or your Provisional Authorised Vaccinator certificate has expired, you can become part of the immunisation workforce via the Vaccinator Foundation Course pathway. Registered and enrolled nurses and nurse practitioners, registered pharmacists are eligible for the Vaccinator Foundation Course.
For any enquiries, please email imaceducation@auckland.ac.nz
BMV training – 27 March 2023
Do you need to quickly find out how to use Book My Vaccine? Do you want to ensure you are fully prepared to manage consumer bookings and appointment schedules? If so, join us for a 30-minute webinar on how to use this powerful tool.
During this webinar, we'll cover the top things you need to know to effectively use Book My Vaccine. Our expert team will give you tips and tricks for managing your schedule efficiently, as well as how to customize your settings to best suit your needs. We'll also share some best practices for communicating with consumers and ensuring they have a smooth and stress-free booking and vaccination experience.
If you're ready to take the next step towards a more organized and efficient scheduling process, then this webinar is for you. Register now to reserve your spot, and join us for this informative and engaging event!
Join us for a 30-minute webinar to ensure you are prepared to use Book My Vaccine to manage consumer bookings and appointment schedules.
Dates and Times
- Thurs Mar 30, 2023 10:30 AM
- Thurs Apr 6, 2023 04:30 PM
- Thurs Apr 13, 2023 06:30 PM
Please register for the series here. Registering will also give you access to the recordings.
Please forward this on to any sites, stakeholders or staff that you feel may benefit from attending this webinar. For additional information on BMV please visit the Te Whatu Ora website.
You can download BMV key messages and reactive QAs here.
Please note, it is likely that your BMV calendar closes on 30th of April.
If you need assistance extending your calendar to the 30th of June, please phone 0800 223 987 or email help@imms.min.health.nz
Holiday vaccine deliveries – 21 March 2023
Due to public holidays, there will be no vaccine deliveries:
- from Friday 7th April to Tuesday 11th April inclusive
- Tuesday 25th and Wednesday 26th April
- Monday 5th and Tuesday 6th June
Please ensure if you have a delivery day on any of those days to order sufficient stock the week prior to tide you over.
Unfortunately, grey cap two and five packs have been delayed; we now expect these to be orderable from 30 March for delivery from 3 April.
This is also a timely reminder to check your holiday hours are correctly listed on your healthpoint. If you need help making changes to this, please email helpdesk@healthpoint.co.nz.
Provider webinar slides and recording – 21 March 2023
Thank you to everyone who was able to attend our provider webinar on Friday, we appreciate it is an incredibly busy time. If you were unable to attend, you can download the slide presentation and recording below.
Vaccinator Bridging Course, Dunedin 4 April 2023 – 21 March 2023
IMAC will be holding an in-person and online vaccinator bridging course on Tuesday 4 April, 9am to 1pm at the Edgar Centre in Dunedin.
The Provisional Vaccinator Pathway is now closed so once your two years of authorisation has expired, it cannot be renewed. Therefore, if you wish to continue vaccinating it is important that you complete a bridging course to become fully authorised. Bridging Training is free until 30 April.
This Vaccinator Foundation Course – Bridging (VBC) is specifically for provisionally authorised vaccinators who wish to become fully authorised vaccinators or pharmacist vaccinators. It builds on Provisional Vaccinator knowledge and skills, bridging the gap between Provisional and full authorisation.
This course will be managed on IMAC’s new learning management system in 2023.
As a provisionally authorised vaccinator, this course will build on your knowledge and skills to enable you to safely and effectively administer vaccines on the National Immunisation Schedule, as appropriate within your scope of practice. It is an essential requirement for authorisation or approval as a vaccinator. Prior to attending a 4-hour practical session, you must complete the online learning which will take 10-12 hours.
Eligibility criteria: this course is available to nurses, pharmacists, midwives and prescribers.
Register on the IMAC website.
NIP and IMAC WEBINAR: Influenza and COVID-19 – 13 March 2023
Get the latest information on the 2023 influenza programme and vaccines, COVID-19 boosters, co-administration and more!
Date: Tuesday 21 March 2023, 5.30pm-6.30pm
Registration: To attend the webinar register here
The NIP team and IMAC will present on:
- Influenza Programme goals for 2023
- Post vaccine safety surveillance
- AIR and Book My Vaccine
- COVID Boosters
- Grey cap COVID vaccines
- Co-administration of COVID and Influenza vaccines
BMV support available – 13 March 2023
For BMV support:
- Weekday drop in sessions from 1pm – 1:45pm. Link to teams meeting: Click here to join the meeting
- The Te Whatu Ora website has a new Book My Vaccine section to support providers who would like to use the tool to offer flu appointments in 2023: Book My Vaccine – Te Whatu Ora - Health New Zealand
- All training material will be located here: https://ncts.my.site.com/cir/s/recordlist/Knowledge__kav/00B4a000000UugVEAS Please note, training material for BMV/NIBs is not yet released (should be out by Monday 13th March)
- Help Desk is also manned through email (help@imms.min.health.nz) and phone (0800 223 987)
New vaccine pack sizes – 13 March 2023
You are now able to order two and five packs of the Infant Pfizer Maroon cap vaccine.
We are expecting two and five packs of the two grey cap formulations to be available to order this week, with delivery from 20th March (TBC).
PROVIDER WEBINAR on 17 March – 8 March 2023
Date: Friday 17 March
Time: 12.30pm - 1pm
Please join the Te Whatu Ora Southern COVID-19 vaccination programme and Medical Officer of Health for Southland and Otago, Susan Jack, for a webinar on the recent transition to the two grey cap Pfizer formulations and upcoming booster eligibility changes. The webinar will include the opportunity for questions, and will be recorded for those unable to attend.
Join on your computer, mobile app or room device
Click here to join the meeting
Meeting ID: 428 268 532 448
Passcode: SVfUvP
Entering a grey cap second primary dose – 7 March 2023
CIR will automatically populate a second dose with the legacy purple cap vaccine if that is the formulation a client had for their first dose. It is important you select "Different Vaccine Administered" and select the new vaccine Pfizer Comirnaty 30 mcg (208) from the drop down list when administering the grey cap second dose.
IMAC WEBINAR: Vaccinating Health Workers and new Stage 2 training – 7 March 2023
Date: Wednesday 15 March 2023, 12-1pm
Teams live event - follow this link to attend the webinar
The webinar is for any potential or current employers of VHWs, their supervisors or VHWs themselves.
The webinar will cover:
1. The Journey of the VHW role so far
2. Detail on the new VHW Stage 2 training
3. Experiences of Māori and Pharmacist providers employing VHWs (TBC)
4. Walk through on how to access the training and the new IMAC Learning Management System (LMS)
5. Where to next for the VHW
6. Questions and answers
IMAC Grey Cap webinar, resources and hot topics – 6 March 2023
Last week, IMAC ran a webinar on the transition to the two grey cap formulations. You can now access the recording here.
IMAC has also prepared a number of resources for the Grey Cap Vaccine which can be downloaded here:
- Comirnaty Grey Cap Factsheet v1 (published 23 February 2023)
- Comirnaty Screening Guide (published 1 March 2023)
- Comirnaty Grey Cap Quick Reference Guide v1 (published 23 February 2023)
- Stop What Top poster for all five Pfizer COVID-19 vaccines (published 15 February 2023)
- Comirnaty Expiry poster (published 15 February 2023)
- Comirnaty Grey Cap Vaccine Record Sheet
Comirnaty Grey Cap vaccines hot topics and updates
- Open vaccine vials have a 12 hours expiry and should be stored in the fridge, as per best practice for all vaccines. This is part of the operations guidance. It can be drawn up and administered cold. Although we are aware that the vaccine is stable at room temperature for up to 12 hours once vial is open, this carries potential risks and is not advised.
- Expiry in syringes: maximum expiry of 6 hours, or the use by time on the vial, whichever is soonest. Syringes can be stored either in the fridge or at room temperature. Clearly mark vaccines with type of vaccine and use by time
- Grey cap vaccine record sheets have been updated - the latest have red text on them.
- Covid Vaccine screening guide has also been updated to v18 - so please use this one
- Spacing and eligibility for boosters, and guidance on spacing post COVID disease has not changed. You will be aware that there will be updated guidance around these issues, but changes only start from 1 April. We will share more information about this in the near future.
NIP Operating Guidelines v.51.0– 6 March 2023
On 1 March 2023, the National Immunisation Programme published version 51 of the operating guidelines. You can find the latest version here, or can download it from the Key Links section at the top of this page.
Summary of changes:
Section A: Ready to vaccinate - summary of changes
|
Version |
Date |
Section |
Summary of Changes |
|
51.0 |
28/02/23 |
Key contacts and Roles and responsibilities |
Updated CIR contact details and hours Added hours for incident inbox Removed ‘Group 1 Employers’ |
|
2.1 |
Updated to add link to Te Whatu Ora website for current IPC advice. |
||
|
2.6 |
Reworded for clarity |
||
|
3.1-3.3 |
Updated to remove outdated information |
||
|
3.7 |
CIR drop in sessions are no longer available |
||
|
7.2 |
Removed reference to alert level settings, updated table 7.2 process step |
||
|
7.3/ table 7.3 |
Ordering site collateral has been updated with current guidance for how to register with BlueStar and what collateral is available to order/ download from Dropbox. |
||
|
Table 8.2 |
Syringe labels table updated with grey cap 30 mcg and 15/15 mcg. Vaccine packs have been updated for reference. |
||
|
Table 8.3 |
Vaccine shelf life table updated to reflect new grey cap vaccines. |
||
|
Table 9.1.5 |
Vaccine unit sizes and dimensions added for new grey cap vaccines, 10 mcg and 3 mcg Comirnaty vaccines have been updated. |
Section B: Pathway to COVID-19 vaccination - summary of changes
|
Version |
Date |
Section |
Summary of Changes |
|
51.0 |
28/02/23 |
17 |
Updated guidance for discontinued Comirnaty 30 mcg purple cap vaccine |
|
18-19 |
New sections with key safety points for Comirnaty 30 mcg grey cap and Comirnaty 15/15 mcg Original/Omicron BA.4/5 grey cap |
||
|
23 |
New section ‘Preparation of doses’ which summarises information previously found in each vaccine section. |
||
|
24.3 |
Removed ‘my vaccine pass’ |
Appendices: summary of changes
|
Version |
Date |
Appendix |
Summary of Changes |
|
51.0 |
28/02/23 |
J |
Updated table to remove outdated information |
February 2023
Grey cap preparedness checklist – 28 February 2023
Firstly, we want to thank you for your continued hard work in supporting our communities to get up to date with their vaccinations.
There will be a few changes in the COVID-19 vaccination programme in the coming months, including the transition from the purple cap to two grey cap Pfizer formulations on 1 March 2023; and the change in eligibility criteria from 1 April 2023.
Earlier this week, IMAC ran a webinar on the transition to the two grey cap formulations. You can now access the recording here.
We acknowledge these are considerable changes and there is a limited amount of time to embed these into our practice to provide a safe and high-quality service. As with any significant operational change, this may lead to an increase in adverse events so extra care must be taken.
To help support a smooth transition, we have sent a preparedness checklist to all site leads which must be returned to Te Whatu Ora – Southern COVID-19 vaccination programme manager, Leanne Brayshaw, by Friday 10 March 2023.
You can also download the checklist here.
Operating Guidelines, COVID-19 policy & consumer collateral – 28 February 2023
The next update to the Operating Guidelines and COVID-19 Immunisations policy will be to support the grey cap rollout, this can be expected online from 1 March on the Ministry of Health website.
The COVID-19 consent form update will be available via the NIP Dropbox. Please ensure you are using the latest version.
The COVID-19 fact sheets (HP8590 What you need to know about the COVID-19 vaccination and HP8591 After the COVID-19 vaccination) are available for pre-order now. If you are not registered with the Blue Star portal, email moh.support@bluestargroup.co.nz and request ‘Vaccine resources access’; include the following details:
- Organisation Name
- Name of person
- Phone
- Physical address
Once the person has been set up as a user. They will receive an email from Bluestar with log on details. Access can take a few working days.
Provider Webinar coming soon – 27 February 2023
The Te Whatu Ora - Southern COVID-19 vaccination team is preparing a webinar for all COVID-19 vaccine providers in the coming weeks. We are finalising the date but will soon circulate an invitation and endeavour to give you as much notice as possible. The webinar will also be recorded for those unable to attend.
The key focus of the webinar will be the transition from the purple cap Pfizer vaccine to the two new grey cap formulations and upcoming changes to eligibility.
Pfizer bivalent vaccine and eligibility – 24 February 2023
From 1 March, a new Pfizer COVID-19 bivalent vaccine will be available to eligible New Zealanders as a booster dose.
- This will replace the existing Pfizer booster and is considered likely to be more effective against Omicron subvariants than earlier vaccines.
- 1.9 million New Zealanders are currently eligible for a first or second booster and will be able to get the bivalent vaccine from 1 March. They will get a significant pre-winter boost to their protection from severe illness, hospitalisation and death from COVID-19.
- We encourage them to take this opportunity in March by visiting www.BookMyVaccine.nz or calling the COVID Vaccination Healthline on 0800 28 29 26 (8am to 8pm, 7 days a week).
From 1 April, an additional booster dose will be made available to:
- anyone aged 30 and over who has completed a primary course, as long as it’s been at least 6 months since their last COVID-19 booster or positive COVID-19 test.
- anyone at increased risk of severe illness from COVID-19 who has completed a primary course, as long as it’s been at least 6 months since their last COVID-19 booster or positive COVID-19 test. These high-risk groups include:
- people aged 65 years and over
- Māori and Pacific peoples aged 50 years and over
- residents of aged care and disability care facilities
- severely immunocompromised people
- people aged 16 years and over who have a medical condition that increases the risk of severe breakthrough COVID-19 illness
- people aged 16 years and over who live with disability with significant or complex health needs or multiple comorbidities.
- health, aged care, and disability workers aged 30 years and over.
1 April is also the start of flu vaccination for 2023. We encourage everyone who is eligible to get their COVID-19 booster and flu vaccination to ensure that they are protected ahead of winter.
Staying up to date with the recommended COVID-19 vaccinations will continue to protect you from the risk of serious illness, hospitalisation, or death from COVID-19. This is particularly important as we approach the winter season.
Supporting messages
- The BA.4/5 bivalent vaccine will replace the existing Pfizer COVID-19 vaccine for boosters. It will not replace the vaccines used for the primary vaccination course, which will continue to be the original Pfizer COVID-19 vaccine or the Novavax vaccine.
- The BA.4/5 bivalent vaccine causes the immune system to create antibodies against both the original variant of SARS-CoV-2 and Omicron subvariants and is therefore likely to provide better protection.
- The additional booster dose can only be administered to eligible people whose most recent COVID-19 vaccine or positive COVID-19 test was at least 6 months ago, irrespective of how many prior doses that person has received.
The Ministry of Health will continue to review new information on COVID-19 and COVID-19 vaccines and will make further recommendations if necessary.
Comirnaty adult vaccine changes: from purple cap to two grey cap formulations – 22 February 2023
The Comirnaty (12+ years) purple cap vaccine is being retired and replaced with two Comirnaty grey cap formulations for use commencing 1 March 2023.
- Comirnaty 30mcg (12+ years) grey cap vaccine for primary doses
- Comirnaty Original/Omicron BA. 4/5 (16+ years, if eligible) grey cap vaccine for booster doses
What you need to know for logistics
Here is how they will appear on the CIR inventory portal:
- Delivery of grey cap vaccines to vaccination providers will commence this week (week starting 20 February). Neither of these vaccines can be used until 1st March 2023.
- Store grey cap vaccines in vaccine fridges prior to use on 1 March. Keep using the Comirnaty (12+ years) purple cap vaccine until 28 February 2023.
- Both grey cap vaccines are prediluted.
- The two new formulations are currently in 10 vial cartons. Planning is underway to repack the vaccine into 5 and 2 vial packs later in March but we have no further information at this stage.
- At the end of the day on 28 February 2023, all Comirnaty (12+ years) purple cap vaccine vials are to be disposed of in the Interwaste vial disposal bins and recorded as wastage in the CIR.
For clinical advice contact IMAC on 0800 466 863.
As the vaccines are prediluted, 100 dose administration kits are not required. Order Unifix 1ml syringes and Vernacare LDS Long Orange 25g 25mm needles (or Verncare LDS Blue 23g 38mm) for administration.
What could catch us out?
- Comirnaty 15/15mcg is only approved for booster use (requires a script if being administered as a primary dose). This is an error if it occurs as it is off-label and will need IMAC clinical support as it may mean the consumer has less “original” in their primary course.
- Comirnaty 30 mcg is intended for primary course only. While a prescription is not required for administering it as a booster dose, the consumer should understand the recommended (first line) booster vaccine in New Zealand is Comirnaty 15/15mcg and the reasons for this. The vaccinator should document this in the notes tab of CIR. This is an error if the consumer has consented for one vaccine and receives a different one and IMAC should be called for clinical advice.
- Grey cap vaccines do NOT require dilution. Dilution of a grey cap vial may mean a consumer receives a dose which contains less antigen than the required dose. This is an error and IMAC should be called for clinical advice. Note: Some reassurance to you – it is almost impossible to add saline to a grey cap Comirnaty vial due to the pressure inside.
What continues to catch us out?
- Vaccines given after expiry date
- Doses being administered before they are “due” – check timings for primary doses and booster doses, these will not change on 1 March with the grey cap transition
- Extra doses being given – never rely on the consumer/admin person. The CIR must be checked – the most frequent error now is an additional second booster being administered
- Use of expired saline
- Demand for vaccines will rise as winter approaches and vaccinating sites need to review their processes and staffing numbers to avoid risk.
What is being prepared to support vaccinators?
- COVID-19 immunisatation policy
- Operating Guidelines
- Consent form updated with clear vaccinator record defining primary and booster doses.
- IMAC webinar on Monday 27 February
If you have any questions or need assistance, please contact Romilly Smith on romilly.smith@southerndhb.govt.nz or 03 476 9915.
IMAC WEBINAR: Comirnaty vaccine changes – 22 February 2023
To help everyone manage the changes above, IMAC will be holding a webinar next Monday. We encourage all providers to attend the webinar, or watch the recording once available.
Date: Monday 27 February 5.30pm-6.30pm (likely shorter)
Register: here
This webinar will cover:
- differences between the vaccines
- vaccine safety and effectiveness data
- practical issues
- resources.
Speakers are:
- Jane Morphet
- Joan Ingram.
The webinar is expected to last 30 min and there will be time for questions at the end.
National Immunisation Programme’s stakeholder hui recording – 21 February 2023
Thank you to those who attended the NIP stakeholder hui held last week.
At the hui Dr Caroline Hart provided a clinical update, then followed by an update from Astrid Koornneef, Interim Director Prevention on our priority work in the Programme including MMR, the Cold Chain Standards Review, Under 5s COVID-19 vaccine for immuno-compromised Tamariki aged 6 months to 4 years, Childhood Immunisation, and Bivalent. We concluded with a presentation on Aotearoa Immunisation Register from the AIR team.
If you missed the hui a recording is available here: Dropbox: NIP stakeholder hui 16 February 2023.
Immunisation Handbook v21 – 15 February 2023
On 15th February 2023, the Ministry of Health published version 21 of the Immunisation Handbook. This has been updated to include the infant Pfizer vaccine for 6-month to 4-year-olds.
You can download a pdf here, or from the Key Links section at the top of this page.
Home visit referrals – 15 February 2023
Our home visit referral pathway has now been updated to include vaccinations for 6-month to 4-year-olds.
If one of your clients is eligible for a vaccination but is unable to access a vaccination clinic (due to disability, a health condition or other reason) please support them to fill out this form or call 03 476 9977 and we'll be in touch to work through a solution.
Updated resources now available – 15 February 2023
New COVID-19 collateral has been uploaded to the NIP Dropbox (Dropbox - National Immunisation Programme – Vaccine resources - Simplify your life), which includes:
- COVID-19 consent form
- What you need to know about the COVID-19 vaccination
- After the COVID-19 vaccination
The two leaflets have been refreshed, along with introducing the maroon cap. There is now no longer a child-specific leaflet to simply things.
Noting two new additions:
- A photograph icon at the top that says “take a photo of this so you can refer to it later” for consumers
- A note at the bottom to say “when is your next vaccine due”
Please ensure you are using the most up-to-date and all consumers are given a copy to take away with them or to photograph for referring to later, and you have disposed of out-of-date collateral.
Please email shelby.davidson@southerndhb.govt.nz to put an order in.
National Immunisation Programme Hui – 13 February 2023
We warmly invite you to the upcoming National Immunisation Programme hui, which is being held from 1.30pm to 2.30pm, Thursday 16 February 2023.
This hui is designed to keep our vaccination and immunisation teams up to date with plans and activities undertaken by the National Immunisation Programme. The hui will include the following presentations:
- Astrid Koornneef Programme update
- Dr Caroline Hart: Clinical updates
- Sarah Keenan: Aotearoa Immunisation Register
If you have specific issues you’d like addressed, please email them to us before Wednesday 15 February 2023.
Please copy the hui invitation details below into your calendar, and feel free to share this invitation with your colleagues who may wish to attend.
Date: Thursday 16 February 2023
Time: 1.30pm – 2.30pm
Link: Microsoft Teams meeting
Click here to join the meeting
Meeting ID: 497 681 435 067
Passcode: iiUXMb
We hope you can join us, but if you can’t we will share a recording of this hui with you.
NIP Operating Guidelines v.50 – 13 February 2023
On Thursday 9 February, the National Immunisation Programme published version 50 of the Operating Guidelines. You can download the new Operating Guidelines here, or find them in the Key Links section at the top of this page.
Summary of changes:
Section A: Ready to vaccinate - summary of changes
|
Version |
Date |
Section |
Summary of Changes |
|
50.0 |
08/02/23 |
7.1 |
Removed COVID19 Tracer app QR code section |
|
Table 9.5 |
Updated Econolog temperature reading device photos to show different colour variations |
Section B: Pathway to COVID-19 vaccination - summary of changes
|
Version |
Date |
Section |
Summary of Changes |
|
50.0 |
08/02/23 |
B |
Section B has been merged with sections C (paediatric Pfizer) and D (Novavax), Comirnaty maroon cap vaccine operational information has been added (pages 81-84). |
|
21 |
COVID-19 vaccine pathway to vaccination section has been refreshed, there is now only one section for this instead of one for each vaccine. |
||
|
21.2 |
Additional sentence added for consumers under observation following concomitant vaccination. |
Section C: Additional Programme guidance, variations and incidents - summary of changes
|
Version |
Date |
Section |
Summary of Changes |
|
50.0 |
08/02/23 |
29.5 |
Updated to reflect Comirnaty maroon cap rollout |
Appendices: summary of changes
|
Version |
Date |
Section |
Summary of Changes |
|
50.0 |
08/02/23 |
Appendix K |
Removed as no longer required |
CIR updates - infant Pfizer – 13 February 2023
As part of the ongoing enhancements to COVID-19 Immunisation Register (CIR), updates were released on Wednesday 8 February 2023.
Record a Vaccination for Tamariki Aged 6 Months to 4 Years: This update enables vaccinators to record a three-dose primary plan of Paediatric Pfizer for eligible tamariki aged 6 months to 4 years old. The eligibility criteria include tamariki who are severely immunocompromised or have complex medical conditions.
Additional notes:
- This is a different plan to the Paediatric Pfizer vaccine used for tamariki aged 5 to 11 years old.
- Care is required when tamariki turn five after their first dose and before completing their primary course. When this occurs, vaccinators will need to administer Paediatric Pfizer for those aged 5 to 11 on the primary plan and record the second and/or third dose using ‘Different Vaccine Administered’ and ensuring that the batch number is entered correctly.
Record Adverse Event from Observation Area: This option has been removed. Adverse events can still be recorded through the consumer’s case where vaccinators will be redirected to a website to complete the form.
Helpdesk: Please continue to direct questions and support requirements to help@C-19imms.min.health.nz or call the team on 0800 223 987. These channels are currently monitored:
- 8am - 5pm, Monday to Friday (from 9.30am on Wednesdays)
- 9am - 2pm, Saturday and Sunday
If contacting via email (recommended), please provide a detailed description of the issue including what your issue relates to, your full name and your mobile number so the resolver can contact you.
Reminder to update Healthpoint – 13 February 2023
Please remember to keep your Healthpoint up to date with your operating hours, what vaccinations you offer, and whether walk in vaccinations are available. This site is used by the programme, and healthline, to direct people to their nearest clinic so it is important that the information is accurate.
If you need assistance, please email helpdesk@healthpoint.co.nz.
Updated COVID-19 vaccination resources – 7 February 2023
The COVID-19 collateral has been uploaded to the NIP Dropbox today (Dropbox - National Immunisation Programme – Vaccine resources - Simplify your life), including:
- COVID-19 Vaccination Consent Form (published 2 February 2023)
- What you need to know about the COVID-19 vaccination (published 2 February 2023)
- After the COVID-19 vaccination (published 2 February 2023)
Please ensure you are using the most up to date versions and all consumers are given a copy to take away with them or to photograph for referring to later.
This collateral is available to download and print, but also available to order from Bluestar in printed tear off pads.
The Ministry of Health website will be updated this week with the updated COVID-19 vaccination policy document and Operating guidelines to reflect the maroon cap (infant Pfizer) updates.
Infant Pfizer: eligibility, referrals and bookings – 7 February 2023
Please share the below information on the rollout of the Comirnity 3mcg 6months-4years vaccine with your colleagues and staff.
In December 2022 Ministers approved use of the paediatric Pfizer COVID-19 vaccine for children aged six months to four years who are at higher risk of severe disease if they were to catch COVID-19. We are pleased to confirm that the vaccine will be available from Thursday 9 February at nominated vaccination clinics.
Eligibility will be limited to children who are severely immunocompromised, or who have complex and/or multiple health conditions which increase the risk of severe disease from COVID-19 (following the Starship Child Health table of underlying comorbidities).
From Thursday 9 February, those with eligible pēpi and tamariki will be able to book appointments through Book My Vaccine or by calling Healthline on 0800 28 29 26.
If you’re not directly offering the vaccine, you still have a critical role to play
The National Immunisation Programme has worked closely with the Districts to identify the specific sites that will be offering the vaccine. In Southern, these will be located in Dunedin, Invercargill, Cromwell, Queenstown and Oamaru.
However, it is possible that eligible children may also present in a variety of other health settings including GPs, pharmacies, Emergency Departments, paediatric departments, oncology departments, children's wards, respiratory outpatients, and beyond.
If your teams do encounter parents or guardians who are enquiring about the vaccine for their eligible children, you can quickly identify the nearest vaccination site for them by visiting Book My Vaccine or calling Healthline on 0800 28 29 26.
If they are unable to access a vaccination clinic (due to disability, a health condition or other reason) please support them to fill out this form or call 03 476 9977 and we'll be in touch to work through a solution. (Note: this form will be updated on 9 February once infant bookings are available)
We will soon be providing resources to support you in conversations with whānau about the vaccination for this age group.
Key messages
- A version of the paediatric Pfizer COVID-19 vaccine is now available for children aged six months to four years who are at higher risk of severe disease if they were to catch COVID-19.
- Eligible children include those who are severely immunocompromised, or who have complex and/or multiple health conditions which increase the risk of severe COVID-19 as detailed in the Starship table of underlying comorbidities
- Parents and guardians of eligible children will be able to book a vaccine by calling Healthline on 0800 28 29 26 or visiting www.BookMyVaccine.nz
- Children aged 6 months to 4 years who are not in these risk categories have a very low likelihood of severe illness from COVID-19 infection and do not need the vaccine (and are not therefore eligible to receive it).
Supporting messages
- The vaccine contains a lower dose of mRNA that has been specially formulated for this age group.
- The vaccine is a three-dose course. The second dose is given 3 weeks after the first dose followed by a third dose at least 8 weeks after the second dose.
- This paediatric Pfizer COVID-19 vaccine for children aged six months to four years was provisionally approved by Medsafe, New Zealand’s regulatory authority, on 1 December 2022. Medsafe provides independent advice to the Ministry of Health. They follow a robust approval process to ensure vaccines meet acceptable standards for quality and that the benefits outweigh any risks. Medsafe only approves a vaccine or medicine for use in New Zealand once it is satisfied that it is of acceptable quality, efficacy and safety.
- Following Medsafe approval, the COVID-19 Vaccine Technical Advisory Group (CV-TAG) recommended to the Ministry of Health that the vaccine be approved for use in children who are severely immunocompromised, or who have complex and/or multiple health conditions which increase the risk of severe disease from COVID-19. This was approved on 12 December 2022.
- The Ministry of Health’s COVID-19 Vaccine Technical Advisory Group will continue to review new information on COVID-19 and COVID-19 vaccines and will make further recommendations if necessary.
For more information about vaccine eligibility; safety and effectiveness; the three-dose schedule; and other aspects of the vaccine rollout, please find a recording of a recent IMAC webinar here.
Updated IMAC COVID-19 vaccine posters – 7 February 2023
With the rollout of the infant Pfizer vaccine beginning later this week, IMAC has updated a number of helpful resources, including:
- Vaccine Safety Poster (published 2 February 2023)
- Stop what top! Poster (published 20 January 2023)
- Vaccine expiry poster (published 20 January 2023)
Infant Pfizer information sheets and guides – 7 February 2023
IMAC has published the following resources for Comirnaty (3mcg) 6 months- 4 years:
- Comirnaty 3mcg maroon cap factsheet (version 1, published 2 February 2023)
- Comirnity 3mcg maroon screening guidance (version 1, published 2 February 2023)
- Comirnity 3mcg maroon cap vaccine preparation guide (version 1, published 2 February 2023)
- Comirnity 3mcg maroon cap schedule (version 1, published 2 February 2023)
COVID-19 Response Recognition Award – 7 February 2023
On December 31 2022, Jacinda Adern announced that groups of frontline workers were to receive a COVID-19 Response Recognition Award, a specific acknowledgement of the service given by so many to New Zealand during the pandemic. This includes MIQ, border, testing, contact tracing and vaccination workforces. Read the full announcement here.
To be eligible for the COVID-19 Response Individual Award you need to have been employed or contracted by Ministry of Health (MOH) or identified as part of the COVID-19 Health and Disability System Response (HSR) for a minimum of 1 month between March 2020 and 30 June 2022 and been active in a role:
- That provided direct operational support to the frontline COVID-19 Health and Disability response; or
- As a Doctor, Nurse or Healthcare and Disability staff who cared for patients with COVID-19, including for example, disability support workers and Māori hauora providers, Pacific health workers
‘Direct Operational Support’ refers to those workers who may not necessarily have faced COVID-19 every day or administered vaccinations but whose roles were critical to ensuring that others could. If you are still unsure whether you are eligible, please contact the team at C19RecognitionAwards@health.govt.nz and we can help.
Contractors, part-time and full-time staff who meet above criteria are eligible to apply for the award.
Please register through the Te Whatu Ora registration portal https://covid-19responseaward.powerappsportals.com/ by 3 March 2023. Registrations will be reviewed against the criteria and awarded after approval.
Awards are a lapel pin in a display box and a personalised certificate. These will be sent to New Zealand addresses.
If you have any questions, please email the COVID-19 Response Recognition Award team at C19RecognitionAwards@health.govt.nz
Read the privacy statement here: Privacy Statement COVID-19 Response Recognition Awards – Te Whatu Ora - Health New Zealand
Universiy of Otago webinar on COVID-19 and Work – 7 February 2023
The University of Otago is holding a full day symposium on COVID-19 and Work, with more than 30 speakers confirmed to share their knowledge and personal experience as to how we can develop more supportive workplaces in a COVID world and help employees impacted by Acute COVID or Long COVID to return to work safely & effectively.
Date: Thursday 23 February 2023
Time: 9:30am – 4:00pm
Venue: Zoom Webinar
Cost: Free, all welcome but RSVP required
You are welcome to join for the full day or, just for the times/speakers you choose. Please register here.
Weather event preparedness – 1 February 2023
Please ensure you have plans in place to move vaccines off-site in case of power failures, ensuring staff are familiar with how to cool chilly bins and use recording devices, and have frozen ice packs ready. This is particularly important to protect against the loss of vaccines in short supply, for example, the mpox vaccine and infant Pfizer vaccine.
January 2023
Change to logistics team operating hours – 30 January 2023
From Wednesday 1 February, the Logistics team will change operational hours to Monday to Friday, 8AM – 5PM, removing Sunday from its operations.
Sunday operations will now happen on the prior Friday, which means:
• Monday deliveries are confirmed Thursday/Friday
• Tuesday deliveries are confirmed Friday/Monday
Please see below a slide on the new supply order cut-off timetable, and how this will impact your ordering:
New date IMAC webinar: COVID vaccine for high-risk under 5yrs – 30 January 2023
IMAC has rescheduled it's webinar on the COVID vaccine for high-risk under 5yrs for Thursday 2 February, 5.30pm-6.30pm.
You must register in order to attend, please register here.
The webinar will also be recorded and we will share this once it is available.
Further information about the webinar:
The vaccine for high-risk under 5-year-olds: Comirnaty (3mcg) 6 months – 4 years, maroon cap, will be available in February.
There is not going to be a specific online course so the webinar will be essential watching for all those administering the vaccine. It will be recorded so you can choose when to watch it.
To administer the vaccine you will need to have completed the online education for Comirnaty (30mcg) purple top and Comirnaty (10mcg) orange top.
Resources are being finalised and we will share those links when available. We will have all the usual information for you including preparation, screening information, fact sheet, and a few posters.
Eligibility will be limited to children who have:
- complex chronic medical conditions including severe neurodevelopmental conditions
- or primary and acquired immunodeficiency
- or are on immunosuppressive therapy such as undergoing treatment for cancer or transplant.
Postponed IMAC webinar: COVID vaccine for high-risk under 5yrs – 24 January 2023
Those who were enrolled to attend the IMAC webinar on COVID vaccine for high-risk under 5yrs on Monday 23 January should have received a notification that the webinar had to be cancelled.
IMAC has yet to release a new date for this, but we will share this with you once it has been confirmed. Please note: it is important to enrol in the webinar in order to attend, and receive updates about cancellations and postponements.
Further information about the webinar:
The vaccine for high-risk under 5-year-olds: Comirnaty (3mcg) 6 months – 4 years, maroon cap, will be available in February.
There is not going to be a specific online course so the webinar will be essential watching for all those administering the vaccine. It will be recorded so you can choose when to watch it.
To administer the vaccine you will need to have completed the online education for Comirnaty (30mcg) purple top and Comirnaty (10mcg) orange top.
Resources are being finalised and we will share those links when available. We will have all the usual information for you including preparation, screening information, fact sheet, and a few posters.
Eligibility will be limited to children who have:
- complex chronic medical conditions including severe neurodevelopmental conditions
- or primary and acquired immunodeficiency
- or are on immunosuppressive therapy such as undergoing treatment for cancer or transplant.
COVID and mpox in 2023 webinar – 24 January 2023
On Tuesday 21 February at 7.30pm, the Goodfellow Unit is hosting a webinar on COVID-19 and mpox in 2023.
The team from the Northern Region Health Coordination Centre will overview what's coming up in 2023 in the covid and monkeypox space, specifically:
COVID-19
- Testing – Anthony Jordan
- Therapeutics – Tim Cutfield
- Vaccines – Anthony Jordan
- Whanau HQ - Anthony Jordan
Mpox
- General update – Teena Mathew
- Management - Jeannie Oliphant
- Vaccines – Anthony Jordan
Please check packing slips for COVID-19 vaccine deliveries – 23 January 2023
DHL has reported a number of CGCs being returned with unopened packing slips. We are concerned if this occurs because it can indicate the goods were not checked as per operating guidelines.
Please ensure you open the packing slip and check the goods and delivery location are correct as outlined in the operating guidelines:
Table 9.5 – site delivery and receipt process: Page 54
Upcoming IMAC events – 23 January 2023
- 31 January: SHIVERS Showcase, 4pm-6pm, Wellington (also via zoom)
The SHIVERS project - Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance - will hold it's annual showcase event in Wellington 31 January from 4-6pm.
International experts include Dr Richard Webby and Dr Paul Thomas - both from St Jude Children's Hospital in Memphis, USA; along with our own stars: Matire Harwood, Sue Huang, Nikki Turner, Cass Byrnes, Peter McIntyre. Find out more here.
- 9 March: Influenza and COVID-19 Symposium, Wellington
More information will be provided soon.
- 16-17 November: Immunisation Conference, Auckland
More information soon, but save the date now!
Selecting the correct sized needle for the deltoid which is used – 23 January 2023
A reminder to all vaccinating sites (particularly Covid sites) that it is important to select the correct sized needle for the deltoid which will be used – larger arms need larger needles.
There has been some online chatter about vaccinators being unaware of this so please ensure clinical leads at sites have provided the appropriate guidance to vaccinators and have a supply of longer length needles.
Planning for infant Pfizer delivery – 18 January 2023
Nationally, we are currently in the planning stages for the rollout of the infant Pfizer for high-risk 6 months to 4-year-olds. There will be a limited supply of the vaccine and the final eligibility criteria are still being determined but will be based on the Starship Child Health table of underlying comorbidities.
The rollout is expected to commence in February 2023 and will comprise three doses, with the second dose given 3 weeks after the first dose, followed by a third dose given at least 8 weeks after the second dose.
Your Project Manager may be in touch to discuss any interest in offering COVID-19 vaccinations to this cohort.
Planning for 2023 – 18 January 2023
As we settle into the new year, now is the perfect time to review your processes and plan for the year ahead.
Ensure your staff are clear on correct procedures, check if there are any further training requirements or renewals (e.g. CPR) due this year and plan ahead to ensure they are booked in.
There is a range of courses available through IMAC that you may find helpful.
Waitangi Day ordering – 17 January 2023
Due to Waitangi day, there will be no regional trucks sent out the evening of Monday 6 February. This means there will be no vaccine deliveries on Tuesday 7 February. Please order 2 weeks of stock the week commencing 30 January. Those providers whose delivery days are Monday and/or Tuesday will need to order by Friday 27 January at 3pm to cover for Waitangi Day and 7 February.
If you have any queries, please contact shelby.davidson@southerndhb.govt.nz.
December 2022
Influenza and COVID-19 symposium 2023 – 21 December 2022
IMAC's annual symposium is coming up in March. For 2023, it is offering a full day symposium from 9am to 4pm on March 9th in Wellington. It will be covering both influenza and COVID-19.
Expert speakers will cover a range of topics including:
- Why Influenza and COVID-19 vaccinations are important
- The National Immunisation Programme (NIP)
- Influenza and COVID-19 internationally
- The 2023 Influenza Immunisation Programme
- The COVID-19 Immunisation Programme
- Influenza and COVID-19 - the immunisation provider’s perspective
- Frequently asked questions regarding influenza and COVID-19 vaccines.
A draft programme will be available soon.
Registrations for in-person and online attendance will open at end of January via our website: immune.org.nz/health-professionals/conference-workshops. We'll let you know more about costs and details closer to the time.
4 pmChanges to ‘Adverse Events Following Immunisations’ webform coming up – 21 December 2022
The National Immunisation Prgramme is updating the webform used to record adverse events following immunisation (AEFIs) for the COVID-19 vaccine. It has been working with the Centre for Adverse Reactions Monitoring (CARM) to improve how New Zealand’s health system captures, records, assesses and reports on adverse effects from any medicines or vaccines. This has led to a review of the systems we use, so we can continue to deliver high-quality medicine safety monitoring capability right across the health and disability sector.
This includes making some changes to the webform used to record AEFIs. Later this month, NIP is upgrading the look and feel of the webform used to log Adverse Events Following Immunisation (AEFIs), to make it easier to lodge these in future. Right now, the webform used to log AEFIs relating to the COVID-19 vaccine is quite heavily tailored to that specific vaccine, and NIP is changing it to be consistent with how all adverse reactions to medicines will be notified in the future. You might notice some of the drop-down fields have different information, but you’ll still use the form in largely the same way as before.
- When will the changes happen? From Thursday 15 December, the webform you use to log any AEFI relating to the COVID-19 vaccine will look slightly different.
- Who does this affect? Anyone who lodges AEFIs via the webform will notice the difference. Anyone who normally uses the CIR system to log AEFIs will be redirected to the new webform.
- Who can tell me more? Please direct any queries to CARMreport@health.govt.nz
Updated collateral in Dropbox – 21 December 2022
Updated booster eligibility criteria collateral for Māori and Pacific translations is now available in our Dropbox. You will find posters, flyers and social tiles for downloading here: Dropbox: Five reasons to get your COVID-19 booster.
Immunisation Handbook v20a – 20 December 2022
On 15 December 2022, IMAC released version 20a of the Immunisation Handbook. Updates have been made to include more information on Meningococcal - MenQuadfi, pregnancy references; Pneumococcal - more information on antimicrobial resistance; smaller updates to HPV, COVID, Measles.
You can access this online here, or download it from the Key Links section at the top of this page.
Paediatric Pfizer for high-risk 6 months to 4-year-olds, likely February 2023 – 20 December 2022
An adapted version of paediatric Pfizer COVID-19 vaccine has been approved for children aged six months to four years who are at higher risk of severe COVID disease eg:
- severely immunocompromised
- complex and/or multiple health conditions
- following the Starship Child Health table of underlying comorbidities.
A further announcement will be made in early 2023 when timing of vaccine delivery is confirmed. It will involve:
- three doses, with the second dose given 3 weeks after the first dose, followed by a third dose given at least 8 weeks after the second dose.
Holiday contacts and hours – 12 December 2022
The Covid Vaccination Programme is running on limited staff over the Xmas/New Year break from 24th December until the 4th January 2023.
During this time if you need to contact someone, we have staff on call:
- 24/12/22 – 31/12/22 Call Lesley Reeves on 0272490412
- 1/1/23 – 3/1/23 Call Romilly Smith on 0272115565 (Romilly will be back in the office from the 4th January)
If you have questions regarding CIR or AIR please email help@C-19imms.min.health.nz.
Please remember to update your healthpoint profile with your holiday hours. If you run into any issue, helpdesk@healthpoint.co.nz
NIP Operating Guidelines v48 – 12 December 2022
On Friday 9 December, the National Immunisation Programme published version 48 of the Operating Guidelines. Section 9.2 has been updated with information and photos to reflect the upgraded temperate monitoring device which will commence being used in DHL deliveries from 28 November 2022.
You can download the new Operating Guidelines here, or find them in the Key Links section at the top of this page.
Recording and presentation slides from the recent vaccine hesitancy and misinformation Webinar – 12 December 2022
On Friday 2 December, the National Immunisation Programme hosted a webinar where Dr Alison Buttenheim from the University of Pennsylvania spoke about behavioural science approaches to addressing vaccine hesitancy and disinformation.
If you missed the webinar, a recording, and the presentation slides are available. As we ran out of time during the webinar to answer all the questions, Dr Buttenheim responded to those questions separately.
The recording, presentation slides, and Q&As can be found in the NIP Dropbox here.
Regional portals and Healthlink re IMAC – 12 December 2022
You can now send a query to IMAC through HealthLink via the HealthLink tab on Practice Management systems for referrals. We welcome queries from health care providers regarding vaccinations, indications, schedule, assessment of possible adverse events and related matters.
For anything requiring an immediate response please continue to call 0800 IMMUNE (0800 466 863). However, if you wish to refer children to Immunisation Outreach please use the Care Connect e-referral form or your local equivalent.
Immunisation handbook v20 – 12 December 2022
On 9 December, version 20 of the Immunisation Handbook was published. You can access this online here, or download it from the key Links section at the top of this page.
This edition has been updated to include the latest on PCV13, second COVID-19 booster recommendation for Māori/Pacific from 40 years and pertussis wording for infants with unstable neurological orders. There are small changes re TB, Varicella and Zoster too.
Medsafe grants provisional approval for two paediatric Pfizer COVID-19 vaccines – 1 December 2022
On Thursday 1 December 2022 Medsafe announced it has granted provisional approval for two Pfizer COVID-19 paediatric vaccines:
- a booster dose of the paediatric Pfizer COVID-19 vaccine for children aged five to 11 years old; and
- the paediatric Pfizer COVID-19 vaccine for children aged six months to four years who are severely immunocompromised.
It is important to understand that Medsafe’s provisional approval does not mean that a decision has been made to use either vaccine as part of our COVID-19 vaccination programme. Instead, the Medsafe approval is only one step in the process. The COVID-19 Vaccine Technical Advisory Group (CV-TAG) is now providing advice to the Ministry of Health to inform a final decision by vaccine ministers on whether to use the vaccines in New Zealand.
We will keep you updated with any decisions, and should one or both vaccines be approved, details of the operational rollout will be shared with you.
Key messages
- Medsafe has granted provisional approval for a booster dose of the paediatric Pfizer COVID-19 vaccine for children aged five to 11 years old.
- The provisional approval is for a booster dose of the vaccine, given at least six months after completing the primary course (this is usually two doses).
- Provisional approval does not mean that a decision has been made to use this as part of the COVID-19 vaccination programme.
- Instead, this is an important step in the process and advice on whether the vaccine should be used will now be prepared by the COVID-19 Vaccine Technical Advisory Group (CV-TAG) to inform a final decision by vaccine ministers.
- The Pfizer COVID-19 vaccine for this age group is an adapted version of the vaccine used for people aged 12 and older.
- Medsafe has also granted provisional approval for the use of the paediatric Pfizer COVID-19 vaccine in children aged six months to four years who are at higher risk of severe disease if they were to catch COVID-19.
- The vaccine for this age group is an adapted version of the vaccine used for five to 11 olds.
- The provisional approval is for three doses of the adapted paediatric Pfizer COVID-19 vaccine, with the second dose given three weeks after the first dose, followed by a third dose given at least 8 weeks after the second dose.
- Advice from CV-TAG to the Director-General of Health has recommended that the eligibility is limited to children who are severely immunocompromised, or who have complex and/or multiple health conditions which increase the risk of severe disease from COVID-19 (following the Starship Child Health table of underlying comorbidities).
- Medsafe approval is only one step in the process. The COVID-19 Vaccine Technical Advisory Group (CV-TAG) is now providing advice to the Ministry of Health to inform Cabinet’s decision whether to use the vaccines in New Zealand.
- CV-TAG's recommendations will then be considered by the Director-General of Health before to a final decision to use is made by vaccine ministers.
- If approval is granted, both vaccines are expected to be available in the first half of 2023.
- Medsafe provides independent advice to the Ministry of Health. They follow a robust approval process to ensure vaccines meet acceptable standards for quality and that the benefits outweigh any risks. Medsafe only approves a vaccine or medicine for use in New Zealand once it is satisfied that it is of acceptable quality, efficacy and safety.
November 2022
Please remember to check CIR and review your processes – 30 November 2022
We have received a number of incidents where consumers have received an extra booster dose (ie a third booster). The contributing factor in all of these cases was a site not using the CIR to check previous doses before administering the vaccine. The CIR should always be used as a live platform wherever possible.
Additionally, we have received several incidents where expired vaccine has been given. Please continue to review your processes and systems to ensure there is a second check of all steps in the vaccination preparation process.
Addressing vaccine hesitancy and disinformation through behavioural change – 25 November 2022
The National Immunisation Programme a webinar on Friday 2 December 2022 from 12.30pm to 1.30pm where Dr Alison Buttenheim will speak about behavioural science approaches to addressing vaccine hesitancy and disinformation.
Dr Buttenheim has served on the National Academies of Sciences, Engineering and Medicine’s Ad-Hoc Committee on a Framework for the Equitable Allocation of the COVID-19 Vaccine, and provided testimony on COVID-19 vaccine hesitancy to the US House of Representatives’ Committee on Science, Space, and Technology.
Click here to join the meeting
Meeting ID: 422 421 309 671
Passcode: 6rMt9U
Dr Alison Buttenheim - bio
- Alison Buttenheim is Professor of Nursing and Health Policy, the Silverstein Endowed Term Chair in Global Women’s Health, and Scientific Director of the Center for Health Incentives and Behavioral Economics at the University of Pennsylvania. Her research addresses persistent behavior change challenges in public and global health.
- Using the techniques and frameworks of behavioral economics, Dr. Buttenheim designs, test, and scales innovative interventions to prevent and mitigate infectious diseases. She is a recognized global expert in the area of vaccine acceptance, with a particular focus recently on COVID-19 vaccines.
- Dr. Buttenheim consulted with several local, state and national governments on the COVID-19 vaccine rollout, and designed and evaluated multiple behaviorally-informed interventions to increase vaccine acceptance, including a financial voucher for older adults in South Africa, a geographically targeted sweepstakes in Philadelphia, and ownership and social norms messaging interventions.
- She has served on the National Academies of Sciences, Engineering and Medicine’s Ad-Hoc Committee on a Framework for the Equitable Allocation of the COVID-19 Vaccine, and provided testimony on COVID-19 vaccine hesitancy to the US House of Representatives’ Committee on Science, Space, and Technology.
- As part of her global health practice, Dr. Buttenheim helped launch and serves as the Behavioural Design Lead for Indlela, a first-of-its kind "nudge unit" supporting innovations in HIV services delivery in South Africa.
DHL upgrade to all-in-one temperature monitoring – 23 November 2022
From Monday 28 November, DHL will be upgrading to an all-in-one tracking/temperature monitor. Please read the following documents for information on the new process.
Reporting Cold Chain Issues – 23 November 2022
If a COVID-19 vaccination incident occurs that includes a cold chain issue, please ensure you inform Immunisation Coordinator in addition to completing a report. This helps the Programme to work with you to ensure robust cold chain processes.
Extending second booster eligibility to Māori and Pacific Peoples aged 40-49yrs – 16 November 2022
Today, the Director-General of Health accepted a recommendation to lower the eligible age for a second booster for Māori and Pacific peoples to 40 years and over.
The second booster will be available to people in this group from Friday 18 November 2022, to be given at least six months after their first booster and at least three months after any COVID-19 infection.
This recommendation was based on clear evidence that the burden on COVID-19 has fallen unevenly, and Māori and Pacific peoples are over-represented in both COVID-19 hospitalisations and deaths with that risk increasing sharply from the age of 40 years. This broader access will help support higher vaccination rates among Māori and Pacific people and provide additional protection.
Prior to the launch of this eligibility extension on 18 November 2022, if a person being treated is eligible, you may consider providing them a second booster in an off-label fashion using a prescription and written consent. IMAC will be able to support any questions that you may have.
From 18 November 2022, once the expanded eligibility has come into effect, all programme collateral, systems and website copy will be updated to reflect the revised eligibility for second booster.
Top-line messages
- From 18 November 2022, Māori and Pacific Peoples aged 40-49 will be eligible for a second booster to provide additional protection against serious illness from COVID-19.
- Second boosters continue to be available for everyone over 50 years old.
- Everyone is encouraged to stay up to date with their recommended vaccinations to protect from the risk of serious illness, hospitalisation or death from COVID-19.
Supporting messages – why (Māori and Pacific focus)
- Māori and Pacific peoples are currently over-represented in both COVID-19 hospitalisations and deaths
- Extending eligibility of second booster doses to include Māori and Pacific Peoples aged 40-49 years will:
- assist in addressing these inequities through supporting higher vaccination rates among Māori and Pacific people.
- expand current eligibility for the second booster by 63,144 Māori and Pacific Peoples (approximately 37% of the eligible Māori and Pacific Peoples aged 40-49 have not yet received their first booster).
- remove the need for those aged 40-49 with undiagnosed comorbidities to get a prescription for their second booster.
- Māori and Pacific Peoples who have not yet had their primary course of COVID-19 vaccinations, or first booster dose, are encouraged to do so.
Supporting messages – why (importance of boosters)
- Staying up to date with the recommended COVID-19 vaccinations will continue to protect you from the risk of serious illness, hospitalisation or death from COVID-19.
- For those who are not at risk of severe illness from COVID-19, a two-dose primary course and a booster dose provides very good and lasting protection against COVID-19.
- The Ministry of Health’s COVID-19 Vaccine Technical Advisory Group will continue to review new information on COVID-19 and COVID-19 vaccines and will make further recommendations if necessary.
- Pre-approved public facing messages from the 2nd booster rollout:
- Keep up to date with your vaccinations
- Keeping up to date with your vaccinations is really important, even if you’ve already had COVID-19.
- You can catch COVID-19 more than once.
- Boosters are the best protection for you and your whānau.
- They can protect you from severe illness, ending up in hospital, and even death.
- That’s why keeping up with your vaccinations is one of the most important things you can do to help protect yourself and those around you.
- It will make the protection you received from your previous vaccination even stronger, and last longer.
- If you’ve had COVID-19 you can get your booster three months after you tested positive.
- Make sure you’re up to date with your COVID-19 vaccinations.
- Check your eligibility at Covid19.govt.nz or call 0800 28 29 26.
- Get your second booster
- Boosters are the best way to protect you and your whānau from becoming severely ill with COVID-19.
- Boosters make sure the protection you have is stronger and lasts longer.
- Keep up to date with your vaccinations
Supporting messages – when
- The second booster will be available for Māori and Pacific Peoples aged 40-49 from 18 November 2022.
- You can only get your second booster six months after your first booster.
- If you have had COVID-19 recently and it is six months after your first booster, you will need to wait at least 3 months after infection before having your second booster.
Supporting messages – how/where
- You can get a booster dose the same way you got your previous COVID-19 vaccinations – including walk-in sites and drive-throughs. You can book an appointment for a booster dose through Book My Vaccine or by calling the COVID Vaccination Healthline on 0800 28 29 26 (8am to 8pm, 7 days a week).
- COVID-19 vaccinations are free for everyone in Aotearoa New Zealand aged 5+.
Te Whatu Ora, National Immunisation Programme webinar 10 November – 9 November 2022
Te Whatu Ora is holding a National Immunisation Programme Stakeholder hui tomorrow, Thursday 10 November 12pm - 1pm, via Teams.
This lunchtime hui is designed to keep our vaccination and immunisation teams up to date with plans and activities undertaken by the National Immunisation Programme. The hui will include the following updates:
- Overall programme updates
- Vaccine updates
- Equity programme updates
We are very pleased to confirm the hui will include a presentation from the team at Kōkiri Hauora and Social services on the great mahi they have been doing in the vaccination service within the community.
Click here to join the meeting Meeting ID: 492 624 400 300, Passcode: hw3bNE
Staff changeovers – 8 November 2022
Please remember to let your local immunisation coordinator know if you have a change in staffing that may affect your vaccination delivery.
Updated myocarditis resources – 8 November 2022
On 2 November, IMAC updated it's webpage on myocarditis: Myocarditis, pericarditis and the COVID-19 vaccine in New Zealand | The Immunisation Advisory Centre (immune.org.nz)
It also released version 6 of the information sheet Myocarditis, pericarditis and the COVID-19 vaccines in New Zealand.
IMAC videos - immunisation during pregnancy and cold chain processes – 8 November 2022
IMAC has added some helpful videos to their website which serve as refreshers for vaccinations. You can find them at the links below:
CIR Helpdesk change of hours – 4 November 2022
From Monday 7 November, the CIR helpdesk hours will reduce to:
- Monday, Tuesday, Thursday and Friday - 8am to 5pm
- Wednesday - 9.30am to 5pm
- Saturday and Sunday - 9am to 2pm
Please check vaccine expiries – 4 November 2022
Please check all Covid Vaccine stock for expiry. Out of date stock needs to be 'wasted' or 'consumed' in the portal and then disposed of accordingly. This includes all vaccine, sodium chloride, and consumables including needles.
Any questions email Romilly romilly.smith@southerndhb.govt.nz
October 2022
NIP operating guidelines v47 – 27 October 2022
On 25 October 2022, the National Immunisation Programme (NIP) released version 47 of the Operating Guidelines. These have been updated in the Key Links section at the top of this page and are available for download here.
Summary of changes:
|
Version |
Date |
Section |
Summary of Changes |
|
46.0 |
25/10/22 |
8.6.2 |
The total allowable transit time of an unopened vial at 2-8 degrees is now 48 hours, previously 12 hours. |
|
9.1.4 |
Updated information about vaccine delivery schedule |
||
|
9.2 |
Removed ‘and security firm’ |
||
|
9.2.2 |
Updated point 3 to include follow in-use expiry on vaccine packs. Due to vaccine expiry extensions, vial expiry may have passed, but the vaccine is still viable |
Section E: Summary of Changes
|
Version |
Date |
Section |
Summary of Changes |
|
46.0 |
25/10/22 |
38 |
Removed section 38 ‘Affected persons under the Vaccinations Order’ |
Appendices: Summary of Changes
|
Version |
Date |
Section |
Summary of Changes |
|
46.0 |
25/10/22 |
B |
Updated delivery information ‘available delivery times’. |
Check packing slips – 27 October 2022
Please ensure you are checking packing slips.
The warehouses have noted several packing slips have been returned unopened with the cold chain packaging. This means the packing slip has not been checked against the contents of the cold chain container/s and consumables received.
It is best practice to confirm:
- The address on the packing slip is yours
- The products and quantities physically received match those listed on the packing slip
Updated COVID-19 consent form – 25 October 2022
There is a new version update of the COVID-19 consent form, this will only be available through the NIP Dropbox for download. As the National Immunisation Programme is anticipating more changes to the consent form, they will not be printing stock for this.
Updates to the consent form include:
- Please let the vaccinator know if you have diabetes
- If you are receiving Novavax, please let the vaccinator know if your first dose was not Novavax
- Minor grammar changes
The updated version of COVID-19 consent form is available from today, Tuesday 25 October 2022 here: Dropbox: COVID-19 consent form
Certificates and authorisations – 25 October 2022
Have you got copies of your education and training certificates? Printing out your certificates so you have a hard copy will be useful later in the year when IMAC updates its Learning Management System.
Keep up-to-date with authorisations
Don't let your authorisation slide - it is essential you are appropriately authorised to vaccinate or play your part in the vaccination process. Guidance on renewing your vaccinator authorisation is available in the Immunisation Handbook Appendix 4.1.3.
Immunisation Handbook v19 – 25 October 2022
On 14 October 2022, the Ministry of Health released version 19 of the Immunisation Handbook. This has been updated in the Key Links section at the top of this page, and can be downloaded here.
You can find an online version of the handbook, and a list of the updates, here: Immunisation Handbook 2020 | Ministry of Health NZ
Updated NIP Incident Notification Form – 20 October 2022
Please find below the latest NIP Incident Notification Form, published 19 October 2022. Completed forms should be sent to judy.walker@southerndhb.govt.nz in the first instance, the programme will then pass the completed form to the Ministry of Health.
What's your risk of death? COVID-19 Mortality report – 20 October 2022
On 12 October 2022, IMAC published an overview of a September Ministry of Health report into COVID-19 mortality. You can find the summary here: https://covid.immune.org.nz/news-insights/whats-your-risk-death-covid-19-mortality-report
Updated IMAC resources – 20 October 2022
This month (October) IMAC released version 2 of the Transit temperature monitoring record for the Pfizer vaccine to reflect the change to allowable travel time for undiluted adult Pfizer vaccine, increased to 48hrs.
Transit temperature monitoring record Pfizer V2
On 10 October, IMAC released version 4 of the Comirnity Pfizer and MMR vaccine comparison chart.
Extension to CIR password expiry – 14 October 2022
From Thursday 13 October, the CIR password expiry timeframes have been extended to 60 days for administrators and 90 days for vaccinators.
This is a change from 28 days for administrators and 42 days for vaccinators.
We appreciate the support of the Districts to work through this issue.
Helpdesk: Please continue to direct any questions or support requirements to help@C-19imms.min.health.nz or call the team on 0800 223 987.
- Please note these channels are currently monitored 8:00am – 5:30pm, Monday – Sunday
- If contacting via email (recommended), please provide a detailed description of the issue including:
- What your issue relates to
- Your full name
- Your mobile number so the resolver can contact you to resolve the issue
Labour day disruptions to vaccine deliveries – 10 October 2022
There will be no deliveries on Monday 24th October (Labour Day) or Tuesday 26th October. If you usually have vaccine deliveries on a Monday or Tuesday, please ensure you order enough stock on the 17th/18th to tide you over for two weeks.
Please also remember to update your healthpoint page if Labour day affects your clinic hours.
Changes to delivery notifications – 10 October 2022
From the close of business on Friday 21 October, NZ Post will no longer send confirmation emails to providers the day before a delivery. They will also cease the 30-minute call ahead from the van.
Updated consent form – 10 October 2022
On 8 September, the Nation Immunisation Programme published an updated COVID-19 vaccine consent form.
Updated consent forms and other resources are also uploaded to the NIP dropbox which you can find in the Key Links section at the top of this page.
Updated IMAC resources: Vaccine comparison chart and post-vaccination advice – 10 October 2022
On the 3rd October 2022, IMAC published version 7 of its COVID-19 vaccine comparison chart. This has been updated to reflect the increased travel restriction time for the Adult Pfizer to a maximum of 48 hours (up from the 12 hours).
COVID-19 vaccine comparison chart V7
IMAC has also added to the following section to the Covid Education section of its website: Post-vaccination advice | The Immunisation Advisory Centre (immune.org.nz)
September 2022
New IMAC hours – 29 September 2022
New hours for the IMAC 0800 IMMUNE (466 863) assistance from 1 October will be:
Monday to Friday 8.30 am to 5.00 pm
Saturday 9.00 am to 1.00 pm (COVID only)
Sunday Closed
- Non-urgent out-of-hours messages will be responded to on the next working day
- As always: dial 111 in emergencies
Peer-reviewed self-assessment form – 27 September 2022
Te Whatu Ora Southern has developed a peer-reviewed assessment process within its mass vaccination centres to ensure all authorised and provisional vaccinators at those facilities are clear on, and comfortable with, every aspect of delivering vaccinations. This includes every step of the process, from the continuity of cold chain and gaining informed consent to safe administration, reporting and dealing with adverse events.
This form has been modelled on an assessment document produced by the Auckland Regional Public Health Service, and we hope it will help you develop an internal auditing system for your own staff.
20 September IMAC webinar recording – 27 September 2022
On Tuesday 20 September, IMAC hosted a webinar entitled 'QAs, Boostrix, Nuvaxovid and more'.
If you were unable to attend, you can watch a recording o their website here.
Cold Chain processes – 27 September 2022
IMAC has created a helpful presentation on cold chain process, including national standards and best practices, common mistakes and what to do following a breach.
Keeping it Cool September 2022
In August, IMAC released version 1 of its Cold Chain Policy Template to assist providers in developing their own cold chain management policy. You can download the template below.
Vaccinator bridging course – 27 September 2022
The Vaccinator Bridging Course is now available through IMAC Learning.
This course is designed to upskill Provisional Authorised Vaccinators (PAVs) to achieve full scope of practice as an Authorised Vaccinator (AV) to administer vaccines on the National Immunisation Schedule (NIS), across the life span or to become a Pharmacist Vaccinator and deliver the scope of vaccines available. Registered/enrolled nurses, pharmacists & midwives are eligible professions for the Vaccinator Bridging Course (VBC).
To complete this course you must be a Provisional Authorised Vaccinator* (provisional authorisation is issued by Te Whatu Ora – Health New Zealand (formally Ministry of Health) and is valid for two years since issue date). Upon completing the education course, you will need to meet all the requirements outlined in Appendix 4 of the Immunisation Handbook to either apply for authorisation or become a pharmacist vaccinator (more details in Authorisation section below). *Please do not register for this course if you have not yet received your provisional authorisation status from Te Whatu Ora – Health New Zealand.
Course delivery and duration
This course involves online learning (10-12 hours), followed by a 3.5 hour in-person tutorial.
Updated Operating Guidelines V46 – 27 September 2022
On Friday 23 September, the National Immunisation Programme released version 46 of the Operating guidelines. A link to this has been added to the Key Links at the top of the page and is available to download here: National Immunisation Programme Operating Guidelines v46.0
|
Version |
Date |
Section |
Summary of Changes |
|
46.0 |
23/09/22 |
9.3 |
Added BD Flu+ 1mL Syringes 23G25mm LDS Needles as an orderable consumable |
Section C: Summary of Changes
|
Version |
Date |
Section |
Summary of Changes |
|
46.0 |
23/09/22 |
Purpose |
Added a key safety point for ageing in changes. Removed outdated information. |
|
23.2 |
Added bullet point to reflect updated ageing in changes |
Section D: Summary of Changes
|
Version |
Date |
Section |
Summary of Changes |
|
46.0 |
23/09/22 |
Purpose |
Added two key safety points for Novavax eligibility extending to consumers aged 12-17 |
|
26 |
Changed 18 years to 12 years |
||
|
Table 29.1 |
Changed 18 years to 12 years Consumers presenting for a booster dose must be 18 years and over |
||
|
Table 29.3 |
Updated sentences for clarity Novavax first and second primary doses can only be administered to consumers 12 years and over Novavax should not be given to pregnant people
|
Section E: Summary of Changes
|
Version |
Date |
Section |
Summary of Changes |
|
46.0 |
23/09/22 |
40.4 |
Updated sentences for clarity |
22 September provider webinar recording and presentation – 23 September 2022
Thank you to everyone who was able to join us for yesterday’s webinar.
Dr Susan Jack, Medical Officer of Health, talked about myocarditis/pericarditis and the importance of covering the risks, symptoms and what a client should do if they experience these as part of informed consent prior to vaccination. We also shared some data insights, the new national booster campaign, and shared an update on the MMR campaign.
If you weren’t able to join or would like to revisit some of the content, please find a recording and the presentation for download below.
Please share this information with your vaccinating staff.
Updated resources – 23 September 2022
IMAC
IMAC has updated its screening and guidance forms to reflect the changes made to schedules yesterday regarding aging-in:
- IMAC Comirnaty Screening and Guidance Form V16 (published 22 September 2022)
- IMAC Nuvaxovid Screening and Guidance Form V6 (published 22 September 2022)
The Novavax information sheet has been updated to reflect the eligibility of those aged 12+ to receive Novavax as a primary course. You can find this here.
IMAC also published version 6 of its vaccine comparison chart on 12 September 2022. You can download that here.
Medsafe
Medsafe has published an updated data sheet for Novavax, now version 6. You can download that here.
Note, this now mentions myocarditis and pericarditis under 4.4 Special warnings & precautions for use:
Myocarditis and Pericarditis
Myocarditis and pericarditis have been reported in male and female adults within 14 days of administering NUVAXOVID (see section 4.8 Undesirable Effects). Available data suggest that the course of myocarditis and pericarditis following vaccination is not different from myocarditis or pericarditis in general. Available data cannot determine a causal association with NUVAXOVID. Vaccinated individuals, parents and caregivers should be instructed to seek immediate medical attention if they develop symptoms indicative of myocarditis or pericarditis such as (acute and persisting) chest pain, shortness of breath, or palpitations following vaccination. The risk of myocarditis and pericarditis after a third dose of NUVAXOVID has not yet been characterized.
Please ensure you are referring to the most up-to-date versions of these documents, and delete any old versions you might have saved.
Logistics update – 21 September 2022
Please encourage your teams and providers to record consumption and keep stock on hand up to date. Consumption of vaccines and consumables is required to:
- Provide local, regional and national view of stock on hand
- Locate stock in the event of a recall or batch trace
- Quarantine Stock
Recording of consumption can be done two ways:
- Entered directly when consumed
- Accounted for as part of daily stock take
Please destroy old expiry/new expiry date info sheets that look like this:
Please let your teams know that the 30 minute call ahead will cease from early November, as security will be removed from the vans.
Extension of Novavax primary doses down to 12 years (currently 18 years) – 21 September 2022
- From 22 September people aged 12 and over can now receive a primary course of the Novavax (Nuvaxovid) COVID-19 vaccine (a primary course is generally 2 doses).
- The extension to those aged 12 years to 17 years (inclusive) is only for a primary course, not boosters. Novavax is only available as a booster for those aged 18 years and above.
- The Pfizer vaccine is the preferred vaccine being used in New Zealand; however, the addition of the Novavax COVID-19 vaccine provides additional choice for 12- to 17-year-olds who have yet to be vaccinated.
- The interval between doses of the primary course is a minimum interval of 3 weeks (same as for Pfizer).
- A prescription will still be required for a second primary dose of Novavax if the first dose was Pfizer. You can get a prescription at a vaccination clinic offering Novavax or prior to your appointment with your preferred GP. Visits to a GP for a Novavax prescription are free.
- Novavax is only available at select vaccination sites.
- Bookings can be made through BookMyVaccine. If you select Novavax, the site will show a list of vaccination centres where Novavax can be given.
Aging-In: minor change for children turning 12 – 21 September 2022
- From 22 September children aged 5-11 would still get a paediatric dose as their first dose, but if they turn 12 before their second dose, they will now receive the adult dose of the COVID-19 vaccine.
- An 8-week gap is recommended between the paediatric and adult doses.
- This change only affects those who turn 12 between receiving their first and second dose of the COVID-19 vaccine. Children under 12 are still only eligible for paediatric doses.
- Children aged 12 and over can receive either Pfizer or Novavax, but a prescription is needed for Novavax. Only Pfizer paediatric doses are available for children aged under 12.
- This change is particularly important for those children with complex health issues or immunocompromised, who are at higher risk from COVID-19, to ensure they are fully protected with vaccine doses appropriate for their age.
CIR
Care is required when recording the second dose for these consumers on the COVID Immunisation Register (CIR) as there is a change in the vaccine on the primary plan. The adult dose of the Pfizer BioNTech vaccine must be selected for the second dose using ‘Different Vaccine Administered’ and the batch number must be entered correctly. See the screenshot below on how to select a different vaccine.
Always check that you have selected the correct vaccine before entering a batch number manually.
For further information:
- Vaccinators guide: https://ncts.my.salesforce.com/sfc/p/4a0000008aXT/a/4a0000002dDf/PBxxqpYKIrNqZp2zxo7gLyfb92ToBam7hO8ei3_0JVM
- The seven rights of vaccine administration: https://www.immune.org.nz/sites/default/files/resources/Written%20Resources/RIGHTS_of_Vax_Admin_A311.03.22.pdf
- The latest COVID-19 vaccine criteria, including age, will help you establish vaccine eligibility with your consumers: https://www.health.govt.nz/covid-19-novel-coronavirus/covid-19-vaccines/recommended-timing-gaps-different-covid-19-vaccines
Helpdesk: Please direct any questions or support requirements to help@C-19imms.min.health.nz or call the team on 0800 223 987.
- Please note these channels are currently monitored 8:00 am – 5:30 pm, Monday – Sunday
- If contacting via email (recommended), please provide a detailed description of the issue including:
- What your issue relates to
- Your full name
- Your mobile number so the resolver can contact you to resolve the issue
Revocation of Health and Disability Vaccination Mandate – 21 September 2022
As announced by the Government on Monday 12 September, the COVID-19 protection framework has been ‘retired’. Of relevance to you is the revocation of the vaccination mandate for the health and disability sector workers (which includes workers in aged care and residential) – that will take effect next week at 11.59pm, Monday 26 September.
The mandate was introduced to protect workers in high-risk settings from COVID-19 and help prevent transmission between workers and vulnerable people. The mandate is no longer needed now the affected workforce has a very high vaccination rate (estimated to be greater than 95 percent), and vaccination has a reduced overall efficacy against Omicron transmission.
The revocation of the order applies to the remaining health and disability workers in the following settings:
- Health practitioners dealing with patients in person, such as doctors, nurses and dentists
- Workers in medical centres/GP practices, pharmacies (such as receptionists or assistants)
- Workers employed or engaged by certified providers – which includes hospitals, rest homes, or residential disability care facilities
- Care and support workers - workers employed or engaged to provide care and support services within a home or place of residence.
All exemptions previously granted will expire at 11:59pm on 26 September. This includes both Temporary Medical Exemptions and Temporary Serious Service Disruption Exemptions. No exemption applications will be accepted after 11:59pm on 26 September.
Some businesses and organisations may choose to continue COVID-19 vaccination requirements through employment contracts - for example, the Te Whatu Ora active vaccination policies across each of the districts and entities will remain in place until a national policy has been agreed. Guidance on employment law, health and safety law and contractual requirements can be found:
Vaccination Spring Fling - coming to you soon! – 21 September 2022
The Southern COVID-19 vaccination programme has identified areas across the region with lower vaccination rates and will be organising a tour of events to support communities in those areas to get up to date with their vaccinations.
Throughout spring and early summer, the programme will be targeting areas with fewer opportunities to get vaccinated, working with the community, local providers and its outreach team.
Clinics will be planned in areas with lower adult COVID-19 vaccine rates, but other vaccinations will also be available. These will vary by location based on clinic capabilities but may include paediatric COVID-19, MMR, influenza and HPV. The vaccines available at each clinic will be advertised once the details are confirmed.
Vaccine deliveries for Labour Day – 20 September 2022
Please note that Labour Day is coming up on the 24th October so there will be no deliveries on that day or the day after, 25th October. Please ensure that, if you have deliveries scheduled for those days, you order enough vaccine the previous week to tide you over until your next delivery day.
Check your saline expiry – 20 September 2022
There have been a number of expiries recently of vaccine and saline, and some expiries coming up at the end of September. Please check your expiry dates and make sure you have not only wasted stock in the portal and also physically disposed of this expired stock accordingly.
Clinic hours and Queen Elizabeth II Memorial Day – 15 September 2022
After the sad news of the Queen’s passing, the Government has confirmed a one-off public holiday on Monday 26 September.
If this affects your clinic hours, please make sure you have discussed this with your project manager and that NIBS has been updated if necessary.
Please also ensure you list any changes to your hours on your healthpoint profile to avoid members of the public turning up for a vaccination when it is not available.
Vaccine deliveries and Queen Elizabeth II Memorial Day – 15 September 2022
After the sad news of the Queen’s passing, the Government has confirmed a one-off public holiday on Monday 26 September.
As per previous Monday public holidays, there will be no deliveries nationwide on Monday 26 September. In Southern there will also be no deliveries made on Tuesday 27 September because NZ Post have confirmed they will not be running regional trucks on Monday evening.
Providers that have designated delivery days of Monday or Tuesday, who will be affected by the outage, are advised to order two weeks of stock on Monday 19th or Tuesday 20th as appropriate.
Please contact Romilly on Romilly.Smith@Southerndhb.govt.nz or 03 4769915 if you have any questions or issues. Out of schedule deliveries may be available if required.
IMAC webinar on 20 September – 15 September 2022
IMAC will be hosting a webinar on Tuesday 20 September, 5pm to 6pm.
Key topics will include:
- Recent changes to COVID vaccine clinical guidance and resources
- Nuvaxovid re myocarditis and pericarditis
- Age appropriate COVID vaccinations
- Benefits of expanding provision of Tdap into Pharmacy settings
- Common questions asked to 0800
- A chance to ask your own questions.
Register for this webinar here.
Updated Novavax Resources – 15 September 2022
IMAC has released version 5a of the Novavax Screening and Guidance form (updated 8 September 2022), and updated the Nuvaxovid factsheet (updated 7 September 2022). You can download these, and the Ministry of Health’s Novavax COVID-19 vaccine policy, below:
Nuvaxovid Screening and Guidance form v5a
Nuvaxovid factsheet v3
Novavax COVID-19 Vaccine Policy Statement Clinical Criteria and Guidance
Please ensure you are referring to the most up-to-date versions of these documents, and delete any old versions you might have saved.
Provider webinar 22 September – 9 September 2022
We would like to invite providers to meet with the Te Whatu Ora Southern COVID-19 vaccination programme team on Thursday, 22 September 12.30-1.30pm.
We will be discussing myocarditis, pericarditis and informed consent; booster data and messaging; an update on the Southern MMR campaign and information about the Aotearoa Immunisation Register.
The session will be recorded for those unable to attend.
Microsoft Teams meeting
Join on your computer, mobile app or room device
Click here to join the meeting
Meeting ID: 495 679 123 636
Passcode: QLt78G
Download Teams | Join on the web
Myocarditis and pericarditis risk with Nuvaxovid – 8 September 2022
Today (Thursday 8 September) Medsafe published a safety alert in relation to the COVID-19 Novavax (Nuvaxovid) vaccine.
Cases of myocarditis and pericarditis were identified in clinical trials of Nuvaxovid (Novavax COVID-19 Vaccine) and a small number of cases have been reported internationally following vaccination. There is a rare but increased risk that these conditions may be present after receiving Nuvaxovid. Post-marketing experience also includes reports of decreased or painful skin sensations.
The medicine sponsor now considers that myocarditis and pericarditis may be rare side effects of Nuvaxovid and the Product Information for Nuvaxovid (Novavax) has been updated to include pericarditis as a potential adverse event.
Healthcare professionals and vaccinators must inform people being vaccinated with either Comirnaty (Pfizer) or Nuvaxovid (Novavax) about myocarditis and pericarditis and advise them to seek urgent medical attention if they experience symptoms.
Any person who experiences myocarditis or pericarditis following either a Comirnaty or Nuvaxovid vaccination should not have a further dose without advice, which is available from IMAC.
For further advice and for plans for the patient’s next vaccination, please call 0800 IMMUNE (0800 466 863) or email 0800immune@auckland.ac.nz
You can read the Medsafe alert, along with advice for consumers and vaccinators here.
You can find IMAC resources on Nuvaxovid here, and information on myocarditis and pericarditis here.
The Ministry of Health has updated its webpages to reflect this change:
https://www.health.govt.nz/covid-19-novel-coronavirus/covid-19-vaccines
https://www.health.govt.nz/covid-19-novel-coronavirus/covid-19-vaccines/covid-19-vaccine-side-effects-and-reactions
https://www.health.govt.nz/covid-19-novel-coronavirus/covid-19-vaccines/covid-19-vaccines-getting-novavax
The downloadable fact sheets and printed material will also be updated accordingly.
Updated COVID-19 chapter in the Immunisation Handbook – 7 September 2022
On 1 September, the Ministry of Health released an updated version of the COVID-19 chapter of the Immunisation Handbook.
Immunisation Handbook: COVID-19 chapter
Please use the online version of the handbook for reference, as opposed to downloading or printing a copy, as this is updated regularly. You can find a live link to the full Immunisation Handbook under the key links section at the top of this page.
Updated Booster Vaccination Policy Statement – 7 September 2022
Please find below the updated COVID-19 booster vaccination policy statement v.7.1, published on 5 September 2022.
Updated operating guidelines v.45 – 7 September 2022
Please find below the latest operating guidelines, version 45.0, published on 5 September 2022, and a summary of changes.
National Immunisation Programme Operating Guidelines v45.0
|
Version |
Date |
Section |
Summary of Changes |
|
45.0 |
5/09/22 |
8.2 |
AstraZeneca removed from ‘Syringe Labels’ table |
|
Table 8.1 |
AstraZeneca removed from ‘Vaccine shelf life’ table |
||
|
8.6.2 |
AstraZeneca removed from ‘Transport restriction duration’ section |
||
|
9.1.5 |
AstraZeneca removed from ‘Unit sizes and dimensions’ section |
||
|
Table 9.5 |
AstraZeneca removed |
Section B: Summary of Changes
|
Version |
Date |
Section |
Summary of Changes |
|
45.0 |
5/09/22 |
Section Guidance |
AstraZeneca removed |
|
15 |
Outdated information about HIS removed |
Section D: AstraZeneca - Removed
Section D: Summary of Changes (previously section E)
|
Version |
Date |
Section |
Summary of Changes |
|
45.0 |
5/09/22 |
29 |
Outdated information about HIS removed |
|
31 |
Outdated information about HIS removed AstraZeneca removed |
||
|
40.1 |
Wording updated for clarity AstraZeneca removed |
||
|
40.7 |
AstraZeneca removed, Novavax added |
Appendices: Summary of Changes
|
Version |
Date |
Section |
Summary of Changes |
|
45.0 |
5/09/22 |
|
Minor wording updates |
|
B |
Pharmacy License expiry option added, as a pharmacy licence holder is deemed to hold CCA for as long as they hold a current pharmacy licence. |
||
|
E |
AstraZeneca removed from cheat sheet |
Check your CPR certificates – 2 September 2022
CPR certificates for vaccinators must now be current. In 2021 the Ministry of Health issued temporary extensions to the expiry date for vaccinator CPR certificates to support vaccinators affected by limited access to CPR training due to Covid-19 disruptions.
The term for the temporary CPR extension has now ended. Jan 1st 2023 is the latest possible expiry date for an extension, however, most will expire much earlier. Check the expiry date of your CPR certificate and if your extension has now expired, you must complete vaccinator first aid training before you can resume vaccinating.
If your CPR certificate is due to expire in the next few months please ensure you book your course in advance and contact your employer if you are unable to access a course within the required timeframe. Please also inform your project manager at the COVID-19 vaccination programme if this affects your site.
Fact Sheets and Data Sheets – 2 September 2022
Please ensure you are keeping up to date with the latest fact sheets and data sheets for COVID-19 vaccines provided by IMAC and Medsafe.
IMAC has released an updated (1 September 2022) fact sheet for Novavax, including information about prescriptions when providing the vaccine as a primary course for under 18s or as part of a mixed course; updated advice on potential responses and adverse events; and revised information on coadministration with other vaccinations.
Please also find below Medsafe's up to date datasheets on Pfizer adult doses, Pfizer paediatric doses and Novavax.
New logistics coordinator – 1 September 2022
Nicole Gorman, Vaccine Logistics Coordinator for the Te Whatu Ora Southern COVID-19 Vaccination Programme, will be leaving the programme on 13 September. We are pleased to announce that Romilly Smith will be taking over this role. Romilly has been working with the programme since early last year so is well versed in the COVID-19 vaccination space and you will be in good hands.
You may start to receive emails/phone calls from Romilly as she takes on more of the role over the next 2 weeks, and please copy her into your correspondence (romilly.smith@southerndhb.govt.nz) to Nicole so that she is across activity. She will also be taking over Nicole's phone so you will be able to reach her on the same number for logistics queries and issues.
Vaccinator training pathways update – 1 September 2022
Vaccinating Health Worker Pathway now open
This is to advise the pathway to become a Vaccinating Health Worker is available for people entering the pathway for the first time. Access to this pathway is via an employer nomination to IMAC. Read more here.
Closure of the Provisional Vaccinator, Provisional Pharmacist Vaccinator and COVID 19 Vaccinator Working Under Supervision
This is to advise the Provisional Vaccinator and Provisional Pharmacist Vaccinator roles will be coming to an end as they were established as temporary measures to help COVID -19 vaccinator efforts during the pandemic.
The last day to enrol in the Provisional Vaccinator Foundation Course will be 1 September 2022. Read more here.
If a person would like to become a vaccinator, there are other options available.
New vaccination campaign material – 1 September 2022
The Ministry of Health recently launched a new campaign to encourage people to get up-to-date with their COVID-19 vaccinations. You’ll start noticing our advertising on TV, radio, print and digital platforms. This includes:
- Māori version: https://youtu.be/mToQwKAmeEE
- English version: https://youtu.be/fEpTqjRRpG4
The ads remind people to get up-to-date with their COVID-19 vaccinations and help answer the ‘why get vaccinated?’ question.
It has also created an interactive digital tool which allows people to enter their own information and find out what COVID-19 vaccination they’re due for. It’s simple, but effective – check it out here. Please feel free to share this with your communities - an embeddable version is coming too.
A range of new materials have been added to the Dropbox, including social media tiles, posters and fliers). This includes a campaign stream designed by Māori for Māori in both English and te reo Māori, and a Pasifika version is coming which will be in Samoan, Tongan and English (this should be loaded within the next couple of days).
(note: some of the posters and flyers will be printed and available shortly via our BlueStar portal)
August 2022
CPR certificate requirements – 24 August 2022
In 2021 the Ministry of Health issued temporary extensions to the expiry date for vaccinator CPR certificates to support vaccinators impacted by limited access to CPR training due to Covid-19 disruptions.
The term for the temporary CPR extension has now expired. From 1st Jan 2023 there should be no vaccinator working with an expired CPR certificate.
If your CPR certificate is due to expire in the next few months please ensure you book your course in advance and contact your employer if you are unable to access a course within the required timeframe.
Adverse Events and recording errors – 24 August 2022
No-one wants adverse events or errors, but they do happen, and when they do, IMAC is available to help if you need it.
If you are dealing with an adverse event, and call 0800 IMMUNE, we encourage you to make a note in the CIR adverse event entry that IMAC was called and note what advice was given. This is to help CARM with their data management, and so they know promptly if IMAC was involved.
Webinars on Vaccinating Health Worker roles – 24 August 2022
IMAC, in partnership with Te Whatu Ora, is providing two opportunities to get an update on the new Vaccinating Health Worker role - one this Thursday and one next Tuesday. They will cover training and authorisation pathways and be followed by an opportunity to ask questions. The session will be recorded for those who are unable to make it. The two options are:
Finally, find out more about Vaccinating Health Worker roles here.
Updated IMAC information: Myocarditis, pericarditis and Nuvaxovid – 24 August 2022
Cases of myocarditis and pericarditis were identified in clinical trials of Nuvaxovid and have also been reported during post-authorisation use. These findings suggest that an increased risk for these conditions may be present after receiving Nuvaxovid. Post-marketing experience also includes reports of decreased or painful skin sensations.
Please ensure you are checking the latest IMAC advice and resources - you can find these linked at the top of this page, as well as a link to sign up for their newsletter.
Recently updated resources include:
- Myocarditis_COVID19-vaccines_for_HP_v5-Aug22.pdf (immune.org.nz) updated 16/8
- Myocarditis, pericarditis and the COVID-19 vaccine in New Zealand | The Immunisation Advisory Centre (immune.org.nz) updated 16/8/2022.
- Nuvaxovid from Novavax | The Immunisation Advisory Centre (immune.org.nz) updated 16/8
- Comirnaty vaccine overview | The Immunisation Advisory Centre (immune.org.nz) updated 17/8
- Vaccinating Health Worker - Supervisor Course | The Immunisation Advisory Centre (immune.org.nz) updated 18/8
Updated operating guidelines V 44.0 – 23 August 2022
Please find below the latest operating guidelines, version 44.0, published on 23 August 2022, and a summary of changes.
National Immunisation Programme Operating Guidelines v44.0
|
Version |
Date |
Section |
Summary of Changes |
|
44.0 |
22/08/22 |
A |
Minor wording updates |
|
3.8 |
Removed point about MIQF |
||
|
8.2 |
New table added for ‘Vaccine Packs’ |
||
|
8.3.1 |
Step 2 Table IMAC regional contacts updated |
||
|
9.1.6 |
Removed 700 dose kits as no longer available. |
||
|
Table 9.3 |
Added all items that can be orders as required. |
||
|
Table 9.4 |
Removed 700 dose kits as no longer available. |
||
|
Table 9.5 |
Changed table title to syringes and needles for prediluted vaccines. Removed MOH Flu+ Syringe 1mL 25Gx1” as no longer available. |
Section F: Summary of Changes
|
Version |
Date |
Section |
Summary of Changes |
|
44.0 |
22/08/22 |
F |
Minor wording updates |
|
43.1 |
Updated information related to ‘Workers subject to the Amended Vaccinations Order’ |
||
|
44.5 |
Information added on large group bookings added |
Appendices: Summary of Changes
|
Version |
Date |
Section |
Summary of Changes |
|
44.0 |
22/08/22 |
|
Minor wording updates |
|
B |
“Vaccine type recorded in NIR” added as a further Vaccine type to accommodate inventory only vaccines that facilities can order, stock, and consume. Boostrix option added to “Vaccine type recorded in NIR field”. |
||
|
I |
Updated NIP incident notification form |
No CIR errors – 23 August 2022
We are pleased to share that this is the first week that, nationwide, no boosters have been incorrectly recorded as additional doses‘.
Please ensure you are not acknowledging CIR warnings without reading them, and are checking the seven rights of COVID-19 vaccination.
Thank you to all of you for continuing to check this process is being carried out correctly.
Second booster eligibility resource – 18 August 2022
We understand that there is some confusion in the community about second booster eligibility when it comes to relevant health conditions. The MoH has provided some clinical guidance around this on its website: Clinical criteria in support of second booster eligibility | Ministry of Health NZ
We have also produced a consumer-facing resource to help the public identify their eligibility, and know where to go for information and support. You are welcome to download and use this in conversations with the public.
Nuvaxovid Screening and Guidance Form – 18 August 2022
IMAC has released an updated Nuvaxovid Screening and Guidance Form (V5). This has been updated in reference to consent forms for boosters, which are no longer required. Consent forms are still required for off-label use such as a mixed schedule or third primary dose.
Vaccination outreach services – 18 August 2022
Te Whatu Ora Southern offers an outreach vaccination service to support those who are unable to access a vaccination clinic.
If you have a patient who requires additional support in accessing a COVID-19 vaccination, please use this referral form or call 03 476 9977 and we will do our best to support their requirements.
Book My Vaccine: Health advice in different languages – 17 August 2022
The Ministry of Health has been working on updates to BMV to enable consumers to access health advice in different languages, including providing contact information for Whakarongorau (who offer language interpreter services in 40 languages) and/or access vaccination support information translated into 28 languages on the COVID-19 website.
The below content will be added to the BMV confirmation screen once the appointment has been completed. This will be implemented on 24 August.
Batch number errors in CIR – 16 August 2022
It should be very difficult to make errors if providers are using their drop-down box option but common mistakes are occurring in the manual entry option. If the batch number is not right then they will come up as not found in the record as per the screenshot below.
Records can be corrected by providers within 24 hours of entry. After this time, please contact Romilly Smith on 03 4769915/romilly.smith@southerndhb.govt.nz, or call/email the helpdesk to make a correction. If they go uncorrected, errors turn up on the Failsafe data quality weekly spreadsheets.
Common mistakes include:
- A capital i instead of a 1
- A Z instead of a 2
- A V instead of a B
- The letter O instead of the number zero 0
- Too many spaces between letters and numbers and no dash
- Using an adult batch number for a paediatric dose
Expiry dates for vaccine, saline and other consumables – 15 August 2022
We are aware that a number of clinics have saline stock that will expire at the end of the month. This is a timely reminder to check the expiration dates of all your consumables, including saline. Please talk to your project manager if you need assistance offloading soon-to-expire stock.
Due to the short expiry date of the last of the 15 packs that were sent out recently, there is a lot of stock expiring this week. Please remember to update your portals once you have disposed of the expired stock. A reminder that Pfizer (12+yrs) can be used up until midnight on the day of expiry. Please see the Ministry’s Operating Guidelines for further detail on this and expiry times for the other vaccines.
Reminder: Update your Healthpoint – 12 August 2022
Please ensure your Healthpoint profile is up-to-date, including:
- If you offer COVID-19 vaccines and what types
- If you offer paediatric vaccines
- Your vaccinating hours (and whether these vary for adults and paediatric vaccinations)
- Whether you provide walk-ins and if they are limited to certain days/times/vaccine types/ages
Healthpoint is an important resource used by the Southern COVID-19 vaccination programme, the Ministry of Health, the national COVID-19 vaccine helpline and the public to find where people can get their vaccinations so it is really important this is kept up to date. This will also help ensure you don't have people turning up when vaccinations are not available.
If you need help, you can reach out to the Healthpoint helpdesk: helpdesk@healthpoint.co.nz
Update on COVID-19 medicines – 12 August 2022
The information for the public on COVID-19 antiviral medicines has been updated on the Ministry of Health website.
The update includes information on eligibility for the medicines, accessing of medicines from your GP or pharmacy, advance prescriptions, and the return of symptoms following treatment.
Te Whatu Ora has also developed an eligibility guide for COVID-19 antiviral medicines to assist. You can find it here: COVID-19 medicines
COVID-19 campaign update – 12 August 2022
The MoH is developing a new campaign primarily focused on boosters including “why get a booster”. It is developing a suite of new content including animated video which will be used on TV and in other channels (including versions developed by Māori and Pacific creative teams).
The first phase of this campaign is in market with radio, print adverts and digital assets. The campaign will appear in mainstream as well as CALD (culturally and linguistically diverse) stations and publications. TV and Out of Home (screens in GPs and health providers) will be live from the w/c 22nd August. In line with the animation, we will be developing new collateral including flyers, posters and online assets. We are planning a mail drop to all householders to be distributed at the end of August.
The second phase will use fresh market research and feedback from Districts, to inform messaging.
Updated consent form – 9 August 2022
The following consent form, published on 13 July 2022, has been updated to include Novavax for second boosters.
HSU dataset change – 5 August 2022
Yesterday the MoH advised Stats NZ had published peer review into Health Service User (HSU) data use for COVID-19 vaccination reporting. You can find more information about the review on this webpage.
They also confirmed that from Monday 8 August, they will be using 2021 HSU data for reporting. While this means a technical decrease in reported vaccination rates overall due to the larger number of eligible New Zealanders being identified, it does not mean any fewer people have been vaccinated. Read the media release here:
Updated health user information informs COVID-19 vaccination programme – Te Whatu Ora | Health New Zealand
COVID-19 Vaccination Booths – 5 August 2022
From today (Friday 5 August) the cost of vaccination booths ordered via Bluestar will no longer be covered by the National Immunisation Programme. The booths will still be available (via the Bluestar portal), but in a ‘user pays’ format. If you wish to order a booth, you will need to email dhbsupport@servicecentral.net.nz.
Delivery to Sites – 5 August 2022
With a higher than normal level of sickness around the motu, there are many new people in the receipting process so it’s timely to remind the providers of the guidelines around receiving vaccine.
Please also remember that the expiry date to refer to is the in-use date on the box (not the vial) so it’s important to always keep the vials with the boxes they came in.
Receiving stock:
- The courier will request the site contact to hand the vaccines over to – refer to the Operational Guidelines linked below to determine the site contact for receipt of stock
- The courier is instructed to hand the goods over ONLY to the site contact
- The courier should never open the vaccine package – Please do not ask them to. If the courier opens the vaccine package, please ask them to stop, and immediately carry on with your normal receipting process, then report it to our team immediately
- The courier is happy to wait for the site contact – if the wait time is longer than 30 minutes, the courier may pick up the goods and take them away for delivery later that day – there will be other providers on the delivery route that need their deliveries
- If the courier is abusive, please report it to our team immediately
- It is never OK to be abusive to the courier
.Please find further advise on the delivery and receipt process in section 9.2 Delivery to Sites in the latest COVID-19 - Operating Guidelines v42.0 (updated 11 July 22).
COVID-19 detecting failsafe reporting guidelines – 4 August 2022
Te Whatu Ora – Health New Zealand has released a new version of its COVID-19 detecting failsafe reporting guidelines, including clarity on the number of days between doses for the detecting failsafe settings and a new update for the data quality failsafe.
COVID-19 Vaccinator Working Under Supervision – 2 August 2022
Te Whatu Ora – Health New Zealand recently announced the COVID-19 Vaccinator Working Under Supervision (CVWUS) training pathway is closing on 11 July 2022, which means there will be no new applications accepted for the programme after this time.
Any staff who are current Authorised CVWUS can continue to vaccinate under supervision until 1 June 2023.
Those that have completed the online course and other training requirements, but have not gained authorisation are recommended to apply for authorisation from Te Whatu Ora – Health New Zealand (click here for the authorisation portal).
For any staff that are enrolled but haven’t progressed with training in the last 6 months, they are recommended to commence the Vaccinating Health Worker (VHW) Stage 1 Training Programme, which will be become available from mid-August 2022.
For authorised CVWUS that want to become VHW, Te Whatu Ora - Health New Zealand will be transitioning to the Vaccinating Health Worker (VHW) role in the meantime.
Please find more about Vaccinating Health Worker training and the transition for CVWUS to VHW on the Health New Zealand website.
If you have staff who are currently in the CVWUS programme and don’t want to become a VHW, they can continue to complete and gain CVWUS authorisation, which will be valid until 1 June 2023.
Concluding the use of AstraZeneca as a COVID-19 vaccination – 2 August 2022
Top-line messages
- The AstraZeneca COVID-19 vaccine is being phased out, in line with the expiry of the remaining stock in New Zealand.
- The last day people will be able to get the AstraZeneca vaccine is 4 September, as the vaccine stock expires on the 5th September. It is still effective if administered on 4 September
- The National Immunisation Programme at Te Whatu Ora will contact people through SMS and email who had AstraZeneca as their last vaccination but haven’t completed their schedule and are eligible to have their vaccinations before 4 September. This message will give them the opportunity to make a booking on or before 4 September.
- Novavax remains as the non-mRNA option for those not wanting or not being able to have a Pfizer COVID-19 vaccine.
- People unable to receive their AstraZeneca vaccination on or before 4 September or who are due their next AstraZeneca vaccination after that, should talk to their doctor or health provider about alternatives.
How will people know this?
- The Ministry of Health website will be updated on 2 August 2022.
- A CPIR campaign (SMS and email) will commence on 3 or 4 August to advise those consumers who are eligible for an AZ vaccine to make a booking and get vaccinated on or before 4 Sept. Please refer to the bottom of this email for the message that will be sent out.
- District Ops Leads have been informed that AstraZeneca will no longer be available after 4 September.
Questions and Answers
How many doses are due to expire in September?
There are around 8300 doses (as of 22 July) of AstraZeneca due to expire on 5 September.
Is there another alternative COVID-19 vaccine for people who don’t want Pfizer?
Yes, there are two effective and safe COVID-19 vaccination options available, Pfizer, and one non-mRNA option– Novavax. People should discuss their options with their health provider.
Are any further orders of AstraZeneca planned?
No further deliveries of AstraZeneca doses to New Zealand are planned following 5 September. Those who have previously received or are eligible for AstraZeneca will have access to the Novavax and Pfizer COVID-19 vaccines.
What about people who’ve booked AstraZeneca after September 5
8 people currently have bookings for AstraZeneca beyond 5 September, the Programme will contact them to let them know they will need to bring their booking forward if they are eligible and let them know there will still be two COVID-19 vaccination options available, Pfizer, and one non-mRNA option– Novavax.
Message campaign – SMS and email to be sent to eligible cohort
AstraZeneca COVID-19 vaccines will no longer be available after 4 September. If you’ve had AstraZeneca but have not finished your primary course or have not had a booster you should consider making a booking on or before 4 September. When booking, be aware if you’ve recently had COVID-19, you must wait 3 months before getting your next vaccination. If you have previously received AstraZeneca, you can still get Pfizer and Novavax vaccines for primary and booster vaccinations. Book an appointment at BookMyVaccine.nz or call 0800 28 29 26 (open 7 days, 8am to 8pm).
Large Group Bookings – 2 August 2022
The large group booking (LGB) function will be introduced to Book My Vaccine (BMV) on the evening of 10 August.
BMV currently supports individual and small group bookings (2 to 5 people). The LGB function will provide an interface for a large group (6 to 30 people) to book together in both the same time slot and across multiple time slots.
In the Southern District, we have turned off this function for all providers in the first instance, please get in touch with your project manager if this is something you would like to offer at your clinic.
If you would like further information about how the LGB bookings work and what details are captured in CIR, please ask your project manager.
July 2022
Changes to Vaccination Certificate and Mask Exemptions payment process in CIR – 28 July 2022
What is happening?
The current manual invoicing process for generating a vaccination certificate domestic, vaccination certificate international, vaccination record, and mask exemption from CIR, is being replaced by an automated process effective 1 August 2022.
Why is this happening?
The payment process for COVID vaccinations was automated to reduce administrative overhead and enable high volume of services. This has now been extended to vaccination records and mask exemptions. The issuing and processing of these manual invoices is a burden for Providers to issue and Sector Operations to process. Shifting to an automated process will remove the need to manually invoice for this service and speed up payment for services.
What do I need to do?
Current – 31 July
For vaccination certificates, records, and mask exemptions that you have generated up to and including 31 July you will still need to invoice manually as per the current process.
1 August - Onwards
For all vaccination certificates, records, and mask exemptions that you generate from 1 August onwards you no longer need to submit invoices. These will now be automatically processed for you.
How will the process work?
- When a document is generated for a NHI, the claim will be created automatically.
- The claims will be processed daily.
- CIR will be updated with the outcome (Acknowledged/Accepted or Negative/Rejected) of the claim daily, and weekly with the associated invoice number.
- An accepted and successfully processed claim will be paid the Friday following the claim period to the bank account associated with the PerOrg ID.
- You will receive a Buyer Created Tax Invoice (BCTI) and Remittance Advice when payment is made via the existing email and postal address associated with the current PerOrg record.
- Even though this process is similar to the Covid Pay Per Dose payments process, the remittance advice for these payments will be separate from the remittance advice from Covid Pay Per Dose. You will be able to differentiate them based on the description of “Covid Doc” for these payments.
- If a claim has been rejected and needs to be corrected, please log a call with the Covid Help Desk (help@C-19imms.min.health.nz) or call the team on 0800 223 987.
If you have any questions about the process?
Please use the Covid Help Desk (help@C-19imms.min.health.nz) or call 0800 223 987
CIR, CICS, NIBS Release 22.10.0 – 28 July 2022
As part of the continuous improvement and ongoing enhancements to the COVID Immunisation Register (CIR), COVID Immunisation Customer Support (CICS) and Book My Vaccine (BMV), new functionality was released on Wednesday evening 27 July 2022.
CIR Enhancements:
There are several enhancements which will support CIR users:
- Flu Vaccination Enhancements in CIR:
- Flu Doses Number Validation: Update to add a limit to the values a CIR user can enter within the dose number field of a flu record. The field will only allow the user to save values from one to nine. This will ensure that unintended invalid values are not entered that may cause GP notifications to fail.
- Enhancement of Person Profile Page: Update to add information to the “Person Profile” page. Last Completed Vaccination, Vaccination Journey Events and Immunisation Activity are now listed on the right side of the Person Profile. Users will no longer need to click through to a patient’s complete COVID vaccination history to view this information.
New additions to Person Profile page:- COVID Immunisation Event list on Profile layout
- Other Immunisation Event (flu) list on Profile layout
- Last Vaccination Date and Last Vaccination Type highlighted on Covid Immunisation and Other Immunisation list views
- AEFI report list on Profile layout
- “Request Document” button on Profile layout
- New Vaccinator Type - Vaccinating Health Worker: Vaccinator type "Vaccinating Health Worker" has been added as an option to the user’s contact record. This will help identify vaccinators that fall under the “COVID-19 Working Under Supervision” group.
- Patient as Consentee Aged 12 and Under: Update to add “Patient” to the dropdown list when entering consent information for a patient aged under 12 years.
Please note: When a vaccinator records the consent of a person under the age of 12, by default, the option will be “Legal Guardian”.
Inventory Enhancements:
There are several usability enhancements for Inventory users:
- New Product - Pertussis Boostrix: Update to create the “Boostrix” vaccine product for Inventory management purposes.
Please note: The “Boostrix” vaccine will not be available to select as a vaccine type for administration.
- “Lockout” National and Regional NZ Holidays for Deliveries: Update prevents IMMS Admins from being able to select delivery dates that fall on a National or Regional holiday that is specified in CIR. Regional holidays are only applicable to Facilities and Sites associated to the region.
Management of the holidays will be completed by a system administrator, and the Logistics team will manage the specification of holiday dates in CIR.
Please note: If attempted approval of a delivery falls on a public holiday, it will be set to the next available delivery date.
- Cut-off times for Providers: Additional cut-off times added in CIR for providers attempting to order a delivery to a Facility or Site. New cut-off times are as follows:
- 3pm Monday for Wednesday delivery
- 3pm Tuesday for Thursday delivery
- 3pm Wednesday for Friday delivery
- 3pm Friday for Monday and Tuesday delivery
Please note: If a delivery is attempted to be ordered outside of the cut-off time, a popup will prompt the user to change the delivery date to the next available delivery day.
- Mandatory Fields for Sites/Facilities: Update to require all mandatory fields for an Active Facility/Site to be completed if a change is made on the record.
BMV Enhancements:
There is one usability enhancements for BMV users:
- Small Group Bookings – Minimum Timing Gaps: Update to add content regarding the minimum timing gap between COVID vaccine doses and a check box for consumers making a small group booking. The check box asks the booking arranger to confirm that they have checked the group’s last vaccination dates and the minimum timing gaps required.
Small Group Booking Bug Fixes:
There are two updates relating to small group bookings:
- Booking Arranger’s Details on CIR Today’s Bookings: An update has been made to correct an issue which was causing the Booking Arranger’s First Name and Last Name details not to display in the CIR Today’s Bookings page, in certain situations.
- Booking Arranger’s Details when Small Group Booking Cancelled: A fix has been applied to correct an issue which occurred when a consumer cancels a small group booking and creates an individual booking. The Booking Arranger’s details from the cancelled small group booking no longer auto-populate to the new individual booking.
CICS Enhancements:
There are several usability enhancements which will support CICS users:
- Disability Services Line (Whakarongorau only): As an extension of Whakarongorau services, the Disability Services Line (DSL) can now record calls as Missed Carer with appropriate call actions also available. Note: this will only be enabled for the DSL team. All other advisors will continue to log calls with existing categories.
- New call category for MMR: Update to add measles-mumps-rubella (MMR) as a Call Category for CICS users. This means advisors can now log calls or emails relating to MMR.
- New Viewing Enrolment Data: New feature that displays consumer GP enrolment information. Users will see the consumer’s enrolment status, the organisation they are enrolled with and the date the enrolment started. This will be visible under the Timeline component on the first page of the Support Case. If the consumer is not enrolled, you will see a heading together with the message No active enrolment information exists for John Doe.
- New Community Services Card (CSC): New feature that displays a checkbox for the community services card. If checked, the consumer holds an active CSC. This is visible on the right-hand-side of the page in the Personal Details section
- Auto Cancel Assistance Tasks: “Special Assistance Tasks” will now automatically close after 90 days. This doesn’t impact the process users follow to support bookings that need special assistance. The auto-complete process will run overnight and clean up tasks that should’ve been closed.
- Viewing Test Results: Update to how test results are displayed. Results within the results tab of an Immunisation Case are now shown in chronological order
Covid-19 and other vaccinations: timings and process – 26 July 2022
A 7-day gap before or after a COVID-19 vaccine is recommended for the Zosatavax shingles vaccine.
A 3-day gap is needed between a Shingrix shingles vaccine or the Fluad Quad flu vaccine and the Novavax vaccine.
All other vaccines may be administered before, after, or at the same time as any other COVID-19 vaccines.
Other vaccines to be given at the same time as BCG Vaccine SSI should not be given into the same arm. If not given at the same time an interval of not less than four weeks should normally be allowed to lapse between the administrations of any two live vaccines. It is advisable not to give further vaccination in the arm used for BCG vaccination for 3 months because of the risk of regional lymphadenitis.
CIR updates – 26 July 2022
The CIR has a new Profile landing page which can be accessed by searching the client, then clicking on their 'Profile Name' under Profiles.
This new landing page will include:
- a new immunisations table displaying all Covid vaccinations in chronological order
- a table displaying 'Other Immunisations', including flu (excluding those administered at GP practices and not entered through the CIR form)
- a new AEFI table.
The new profile landing page will help vaccinators to more quickly assess whether someone is eligible for a vaccination. All the information they need should be on the new profile page.
Second boosters and early requests – 22 July 2022
Reminder: Six months has been set in Book My Vaccine at 185 days. The failsafe, for when a vaccine is considered “too early” is set at <180 days (ie 179 days or less). This is the lowest possible time six months can be in days, if we assume the day of the previous vaccine is day 0. This also gives a 5-day window for those who present early as walk-ins. Unfortunately, the failsafe number (180) was accidentally taken as the six-month number (185) and thus the confusion about the 4-day leeway.
If someone wants to get their booster earlier than 180 days, you need to consider: Is the person able to, and likely to, come back easily when booster is due?
YES: If so, ask them to return on or after the 180-day mark.
NO: If not, get case-specific clinical advice from IMAC to help you make a clinical decision on the appropriateness of giving an early vaccination. You can then explain any additional risks to the consumer.
IMAC has lots of resources online and will be adding to these to help with these conversations.
You will also need to make a note in CIR recording the rationale for giving an early vaccination. This should include comment about why it’s difficult for the person to return at a later date. For example, that might involve travel costs, work hours, equity issues, time or family commitments; or that the clinic is only available in their community for a few days. You need to be confident you are doing the best for the particular patient given their particular circumstances.
Outreach events, mobile clinics, and prescribers
Regions who are planning outreach events or similar clinics when it is expected that there may be several requests for 'early' doses should try to have a prescriber available to provide scripts in a way that suits the Operation.
Booster Vaccinations Policy Statement – 22 July 2022
Please familiarise yourself with the updated Booster Vaccination Policy Statement which includes details on different vaccine types and intervals for first and second boosters.
Expiry dates for Comirnity and Nuvaxovid: posters for fridges – 21 July 2022
As you may know, expiry dates have been updated for Comirnaty (adult and paediatric) and Nuvaxovid and the updated dates should now be on the boxes. To help ensure people use the expiry date on the box, and not the one on the vials, IMAC has developed some posters. We encourage you to pop these posters on your fridges or where you think they will help remind people.
Vaccine incident and cold chain resources – 21 July 2022
IMAC has recently added a couple of forms (which can be filled in online) and a flow chart on cold chain incidents.
Where to go for help – 15 July 2022
The CIR helpdesk (0800 223 987) is available to help you with any CIR advice, including how to enter a second booster into CIR and how to enter an overseas dose or advice.
For clinical advice please continue to go to IMAC.
AstraZeneca and Novavax approved for second boosters – 15 July 2022
Top line messages
- From 14 July people 18 years and older will be able to access second boosters for both AstraZeneca and Novavax via Book My Vaccine.
- The addition of AstraZeneca and Novavax means people now have a choice in their second booster vaccination, with the Pfizer second booster already available.
- For the Novavax second booster NO prescription is needed
- For the AstraZeneca second booster a prescription IS needed.
Supporting messages - what this means from a consumer perspective
Novavax
- You need to be 18 years and older to access the second booster for Novavax
- Novavax second booster does not require a prescription at a minimum dosing interval of 6 months
- Novavax first booster does not require a prescription at a minimum interval of 6 months (As implemented last week)
- Novavax primary course (mixed schedule) requires a prescription (no change from current)
- Novavax primary course (both Novavax) does not require a prescription (no change from current)
- The second booster of Novavax can be administered after a primary course and third dose of any approved COVID-19 vaccine.
AstraZeneca
- You need to be 18 years and older to access the second booster for AstraZeneca
- AstraZeneca second booster requires a prescription at a minimum dosing interval of 6 months
- AstraZeneca first booster requires a prescription at a minimum dosing interval of 3 months (no change from current)
- AstraZeneca primary course (mixed schedule) requires a prescription (no change from current)
- AstraZeneca primary course (both AstraZeneca) does not require a prescription. (no change from current)
Supporting messages – why
- A second booster is important for our most vulnerable people as we move into the winter peak.
- Staying up to date with the recommended COVID-19 vaccinations will continue to protect you from the risk of serious illness, hospitalisation or death from COVID-19. This is particularly important as we approach the winter season.
Supporting messages – when
- You can only get your second booster six months after your first booster.
- If you have had COVID-19 recently and it is six months after your first booster, you will need to wait at least 3 months after infection before having your second booster.
Supporting messages – how/where
- AstraZeneca and Novavax are both only available at select vaccination centres with bookings needing to be made through Book My Vaccine. If you select AstraZeneca or Novavax, the site will show a list of vaccination centres where they can be given. Not all sites are able to deliver these vaccines.
- The Pfizer COVID-19 vaccine remains the preferred COVID-19 vaccine for use in New Zealand, reflecting its excellent safety and effectiveness profile. Talking to your GP or health professional will help in determining the best vaccine booster option.
Q&A
Why do you need a prescription for an AstraZeneca booster but not Pfizer or Novavax?
AstraZeneca is approved for use in New Zealand only as primary series vaccine (eg the first two doses) and has not been approved by Medsafe as a booster. It is therefore only available on prescription as an off-label medicine. Pfizer and Novavax have been approved by Medsafe as first boosters, and the Director-General of Health has authorised them as second boosters under section 34A of the Medicines Act, so they can be administered without prescription.
Reminder: COVID-19 and other vaccinations – 15 July 2022
If you’re having a Zostavax shingles vaccine, a 7-day gap before or after a COVID-19 vaccine is recommended.
For Novavax, a 3-day gap is needed for if you’re having a Shingrix shingles vaccine, or the Fluad Quad flu vaccine.
All other vaccines may be administered before, after, or at the same time as any other COVID-19 vaccines.
Six-month interval for second booster – 11 July 2022
The Director General’s order advises that the second booster can only be administered six months or more after a consumer's last dose. We understand some people have been advised that it can be given up to four days early but this is incorrect. If the vaccine is given before the 6-month gap, then this must have a prescription.
The shortest time period 6-months can be is 181 days. Thus, the CIR failsafe is set at 180 days. Anything 180 days or less must be reported.
If the patient needs their second booster early, it can be provided on prescription from the consumer’s doctor (e.g., if the doctor has advised an early vaccine pre-surgery or similar).
Reminder: Informed consent process – 8 July 2022
Informed consent is required for all vaccination events – this includes discussing possible reactions and what to do / where to go if these are experienced.
This is not being done in all situations. In some cases, this is due to not wanting to put the consumer off being vaccinated, or is being missed during second or booster doses because of an assumption that the consumer has already had this information.
A written post-vaccine handout (Getting your COVID-19 vaccine: What to expect) is recommended as part of the consenting process. Sites should ensure they have access to the latest leaflet and offer it at all vaccination events. You can request this collateral through romilly.smith@southerndhb.govt.nz
We recommend vaccinators watch the video on informed consent, myocarditis and pericarditis which is available free through the online IMAC e-learning page.
You and your staff may find it helpful during quieter times to role play the consenting process and other common scenarios to make sure everyone is comfortable with the required steps.
CIR logins – 8 July 2022
Please remember to log in to CIR at regular intervals, and check that your login is active before any planned activity. Any staff who have not logged into CIR for 60 days will need to have their access requalified and reinstated by emailing shelby.davidson@southerndhb.govt.nz.
MoH Second booster communications – 8 July 2022
The Ministry of Health has launched targeted outreach to Māori and Pacific people aged 50+, and other ethnicities aged 65+ who are eligible for a second booster. This includes email and SMS, followed by outreach calls and a posted letter to those they don’t hold email/phone details for.
These outreach messages include information on why people should get a booster and the fact that they can get their flu vaccination at the same time. Additionally, they will be including the consumer's nearest clinic, and how they can find others in their area. Please note that this information is being sourced through healthpoint so this is another timely reminder to make sure that your profile is up to date.
The data has been cleaned for a number of factors, including people who have already had their second booster, are not yet eligible due to the date of their last vaccine, have recorded having COVID-19 in the last 3 months or have previously opted out of communication.
A radio campaign is also underway promoting second boosters.
Pfizer vaccination journey - user guide – 6 July 2022
With increasing changes in eligibility for different vaccinations, and different dose intervals, we understand that it can get a little confusing for the public so we have created a series of flow charts and information sheets to help people identify where they are in their vaccination journey, and if/when they are eligible for other doses.
Please feel free to download and use these when talking to your clients: COVID-19 Vaccination Journey
Important: Update your Healthpoint profile – 6 July 2022
Please ensure your Healthpoint profile is up-to-date, including:
- If you offer COVID-19 vaccines and what types
- If you offer paediatric vaccines
- Your vaccinating hours (and whether these vary for adults and paediatric vaccinations)
- Whether you provide walk-ins and if they are limited to certain days/times/vaccine types/ages
Healthpoint is an important resource used by the Southern COVID-19 vaccination programme, the Ministry of Health, the national COVID-19 vaccine helpline and the public to find where people can get their vaccinations so it is really important this is kept up to date. This will also help ensure you don't have people turning up when vaccinations are not available.
If you need help, you cant reach out to the Healthpoint helpdesk: helpdesk@healthpoint.co.nz
Novavax booster approved – 5 July 2022
From 5 July, a free Novavax booster is available without a prescription to those over 18, at least six months after completing their primary course of any COVID-19 vaccine (Pfizer, Astra Zeneca or Novavax) at select vaccination centres around the country. This gives everyone over 18 years old a choice in which COVID-19 booster vaccine they receive.
- The Government has agreed the use of the Novavax COVID-19 vaccine Nuvaxovid as a booster without a prescription, giving people more choice and more opportunity to receive a COVID-19 vaccine booster dose.
- This decision follows Medsafe’s update to the provisional approval, to include use of Nuvaxovid as a booster after the primary course of any of the COVID-19 vaccines available in New Zealand.
- Novavax is not yet available on the programme as a second booster. However, you can discuss your situation with your healthcare provider, and they can provide a prescription if appropriate.
- A prescription is still required for those seeking Novavax as a second dose after a first dose of Pfizer or AstraZeneca.
Staying up to date with the recommended COVID-19 vaccinations will continue to protect you from the risk of serious illness, hospitalisation or death from COVID-19.
Q&As Novavax vaccine (Nuvaxovid) booster
Can I get a Novavax COVID-19 vaccine booster if I had a different vaccine as my primary dose?
Yes. Novavax is approved for use as a booster after a primary course for any of the COVID-19 vaccines available in New Zealand – Pfizer, AstraZeneca and Novavax for 18 years and over.
What age do people need to be to get a Novavax COVID-19 vaccine booster?
18-year-olds and above can get a Novavax COVID-19 vaccine booster.
How long do I have to wait after a primary course to get the Novavax COVID-19 vaccine booster?
You can get the Novavax COVID-19 vaccine booster six months after completion of the primary course (for most people, this is two doses).
Why is the Government offering Novavax COVID-19 vaccine as an option now?
It’s important that everyone who is eligible for a booster, gets a booster. However, we recognise that the Pfizer COVID-19 vaccine booster isn’t suitable for everyone, so we want to be able to provide another option.
The Pfizer vaccine remains the preferred COVID-19 vaccine for use in New Zealand. Staying up to date with the recommended COVID-19 vaccinations will continue to protect you from the risk of serious illness, hospitalisation or death from COVID-19. This is particularly important over winter.
Do I need a prescription?
A prescription is not required for a Novavax booster if you are over 18 and it is at least six months since completing your primary course of any of the COVID-19 vaccines available in New Zealand –from Pfizer, AstraZeneca and Novavax.
How safe is the Novavax COVID-19 vaccine booster?
Novavax like all vaccines can cause side effects in some people. These are usually some normal side effects from any vaccine such as high temperature, sore arm and headache, see the Consumer Medicine Information https://www.medsafe.govt.nz/Consumers/CMI/n/Nuvaxovid.pdf
Can Novavax be used by pregnant people?
The Pfizer COVID-19 vaccine remains the preferred choice of vaccine for this group. If this vaccine is not suitable for you, please talk to your healthcare provider about alternatives.
How can I get a Novavax COVID-19 vaccine booster?
If your second dose was six months ago you can get your Novavax booster, which is offered at select vaccination centres. Bookings can be made using Bookmyvaccine.nz or call COVID Vaccination Healthline 0800 28 29 26 (8am - 8pm, 7 days a week).
I am eligible for a second booster – can I have Novavax?
Pfizer is recommended for anyone who is eligible for a second booster as it is effective/safe etc. The Ministry of Health will continue to monitor the research around Novavax as a second booster. You can discuss your situation with your healthcare provider, who can also utilise IMAC for clinical support.
How many people do you expect to get the Novavax COVID-19 vaccine booster?
It’s unclear at this stage how many people will request the Novavax booster.
Vaccine mandates to end for some health and disability sector workers – 1 July 2022
From 11.59pm on Thursday 7 July, workers who are not public facing in certain healthcare environments and/or do not provide healthcare services directly to patients, will no longer be required to be vaccinated against COVID-19. This means Government vaccine mandates for some health and disability sector workers are being removed.
Who is still mandated?
- Health practitioners dealing with patients in person, such as doctors, nurses and dentists will continue to be covered by the Vaccinations Order.
- Workers in medical centres/GP practices and pharmacies (such as receptionists or assistants) whose role involves being within two metres or less of a health practitioner or a member of the public for a period of 15 minutes or more.
- Workers who are employed or engaged by certified providers – which includes hospitals, rest homes, or residential disability care facilities - and who, as part of their ordinary duties, come within two metres or less of a health practitioner or a person to whom health care services are provided for a period of 15 minutes
Who is not mandated?
- Workers who are not public facing in certain healthcare environments and/or do not provide healthcare services directly to patients. This includes back-office staff who, for example, do the accounts for a GP and groups like the police and firefighters who may be first responders in the course of their duties but providing health services is incidental to their core work.
Key messages
- The Government is changing the COVID-19 Public Health Response (Vaccinations) Order 2021 as it relates to health and disability sector workers.
- The Government is limiting the workers who must be vaccinated against COVID-19 to those who face a higher risk of contracting and transmitting COVID-19 in the workplace than in the wider community.
- From 11:59 pm 7 July, workers who are not public facing in certain healthcare environments and/or do not provide healthcare services directly to patients, will no longer be required to be vaccinated against COVID-19. This includes back-office staff who, for example, do the accounts for a GP and groups like the police and firefighters who may be first responders in the course of their duties but providing health services is incidental to their core work.
- Vaccination against COVID-19 continues to be highly recommended for all health and disability sector workers – including those in the aged care sector.
Supporting messages
- Workers in medical centres/GP practices and pharmacies (such as receptionists, assistants) and whose role involves being within two metres or less of a health practitioner or a member of the public for a period of 15 minutes or more will be covered by the Vaccinations Order.
- Health practitioners providing health services to patients in person including doctors, nurses and dentists will continue to be covered by the Vaccinations Order.
- Workers who are employed or engaged by certified providers – which includes hospitals, rest homes, or residential disability care facilities - and who, as part of their ordinary duties, come within two metres or less of a health practitioner or a person to whom health care services are provided for a period of 15 minutes or more will continue to be covered by the Vaccinations Order.
- These decisions have been made because requiring vaccination is no longer considered a proportionate response to the risk now posed by COVID-19. This is a result of high vaccination rates, the changing nature and perception of risk, and a significant portion of the population now having contracted and recovered from COVID-19.
- When reviewing whether health and disability sector workers should still be required to be vaccinated against COVID-19, the Ministry of Health considered workers across the sector based on the different workplaces and the ongoing level of risk in those environments.
- The Ministry of Health considers there is still a high risk for workers who work directly with people who may have COVID-19 or people who are at high risk of severe outcomes from COVID-19 and for those who work directly in aged care or with vulnerable and/or disabled people.
- The risk level in different workplaces has been considered using these criteria:
- the nature of the work and the work setting, in terms of the risk of exposure to COVID-19 compared to being in the broader community
- the nature of the work and the work setting and the interaction with people who are at greater risk of severe disease should they contract COVID-19.
BMV and second boosters – 1 July 2022
Thank you for your patience as BookMyVaccine is further developed to make the booking of second boosters a smooth process.
- Whilst the correct interval between first and second boosters is 184 days, the system requires an extra day in order to sync with CIR. Therefore, it will allow the user to book 185 days from the inputted date of their last dose. CIR continues to calculate by calendar months so if a patient presents 6 months after booster 1 the vaccinator will not see an early warning screen and can go ahead and vaccinate.
- MoH is monitoring incorrect bookings - about 80% of the errors are user error - and are working to text users with early bookings advising them they need to amend their booking.
- We are aware that some users have been selecting the "booster" option rather than "second booster" option in BMV which allows them to book at a three month interval instead of six. To reduce this confusion, these fields are being edited to read "first booster" and "second booster".
- We have also been made aware that interval warnings are not appearing in CIR for group bookings so please ensure you are checking the individuals eligibility.
We have received feedback on a few issues which have been passed on to the Ministry of Health for investigation. If you do come across any issues or concerns, please talk to your project manager.
June 2022
Processes for providers: second booster eligibility – 28 June 2022
As further COVID-19 vaccination doses are rolled out, it is increasingly complex for consumers to keep up to date on how eligibility criteria apply to their specific situation. We have also seen instances of people ticking the wrong booster box in BookMyVaccine allowing them to book within a shorter interval.
In line with national COVID vaccination provider standards and policies, all COVID vaccination providers should review their processes and update these for managing the delivery of the second booster role out.
To minimise disruption for both consumers and vaccination providers we recommend you implement a process for reviewing the below prior to consumer attendance at the clinic for a second booster and, if necessary, making contact to rearrange their appointment:
- Check CIR to confirm the booking will be a minimum of 6 months after a consumer received a first booster. If, for a specific clinical reason, the second booster dose is being administered before the minimum 6-month interval, a prescription is required which must be sighted by the vaccinator and a copy uploaded to the CIR. Informed consent must be written and a copy uploaded into the CIR.
- Check if the consumer has had a COVID-19 infection and that three months have passed since recovery before administering the second booster (or any COVID-19 vaccination). If a specific clinical reason exists for the administration of a second booster before this 3-month interval, a prescription is required which must be sighted by the vaccinator and a copy uploaded to the CIR. Informed consent must be written and a copy uploaded into the CIR.
The updated consent form has now been uploaded to the dropbox.
Second boosters to commence from Tuesday 28 June – 27 June 2022
The Ministry of Health has today confirmed the rollout of second boosters for those at risk of severe illness from COVID-19. This will come into effect from Tuesday 28 June and until then remains an off-label dose. We are aware that some members of the public may be seeking a second booster today following the announcement, however, scripts for second boosters are still required until tomorrow.
Thank you for your ongoing patience through this process and your ongoing work to protect our community during what we know is a very busy time for all of you.
Second booster eligibility
A second booster is recommended for those at increased risk of severe illness from COVID-19 – a minimum of 6 months after a first booster. For those who are not considered at risk of severe illness from COVID-19, a two-dose primary course and a booster dose provides very good protection against severe illness from COVID-19.
The following people are recommended to receive a second booster as a priority:
- people aged 65 years and over
- Māori and Pacific peoples aged 50 years and over
- residents of aged care and disability care facilities
- severely immunocompromised people who received a three-dose primary course and a fourth dose as a first booster (noting this would be a fifth dose for these people)
- people aged 16 years and over who have a medical condition that increases the risk of severe breakthrough COVID-19 illness and
- people aged 16 years and over who live with disability with significant or complex health needs or multiple comorbidities.
In addition, a second booster is available for:
- all people aged over 50 years
- health, aged care and disability workers aged over 30 years.
Please note: a second booster is not recommended for healthy pregnant people.
People will be able to book their second dose through BookMyVaccine from tomorrow, Tuesday 28 June.
Further information
The policy statement outlining the use of second boosters will be published on this webpage this afternoon. Likewise, the amended consent form will be on our Dropbox shortly.
The immune and medical conditions where a second booster is recommended are listed below. People in these groups are likely to have an ongoing increased risk of severe COVID-19 even after primary vaccination. These examples are not exhaustive, and providers may include individuals with conditions similar to those listed below, based on clinical judgement.
|
Category |
Examples |
|
Immunocompromising conditions |
|
|
Cancer |
Non-haematological cancer including those diagnosed within the past 5 years or on chemotherapy, radiotherapy, immunotherapy or targeted anti-cancer therapy (active treatment or recently completed) or with advanced disease regardless of treatment. Survivors of childhood cancer. |
|
Chronic inflammatory conditions requiring medical treatment with disease modifying anti-rheumatic drugs (DMARDs) or immune-suppressive or immunomodulatory therapies. |
Systemic lupus erythematosus, rheumatoid arthritis, Crohn’s disease, ulcerative colitis, and similar who are being treated. |
|
Chronic lung disease |
Chronic obstructive pulmonary disease, cystic fibrosis, interstitial lung disease and severe asthma (defined as requiring frequent hospital visits or the use of multiple medications). |
|
Chronic liver disease |
Cirrhosis, autoimmune hepatitis, non-alcoholic fatty liver disease, alcoholic liver disease. |
|
Severe chronic kidney disease (stage 4 or 5) |
|
|
Chronic neurological disease |
Stroke, neurodegenerative disease (e.g dementia, motor neurone disease, Parkinson’s disease), myasthenia gravis, multiple sclerosis, cerebral palsy, myopathies, paralytic syndromes, epilepsy. |
|
Diabetes mellitus requiring medication |
|
|
Chronic cardiac disease |
Ischaemic heart disease, valvular heart disease, congestive cardiac failure, cardiomyopathies, poorly controlled hypertension, pulmonary hypertension, complex congenital heart disease. |
|
People with disability with significant or complex health needs or multiple comorbidities which increase risk of poor outcome from COVID-19 |
Particularly those with trisomy 21 (Down Syndrome) or complex multi-system disorders. |
|
Severe obesity with BMI ≥ 40 kg/m2 |
|
|
Severe underweight with BMI < 16.5 kg/m2 |
|
Second booster and flu eligibility announcement – 23 June 2022
We have been advised that there will be an announcement on Monday 27 June that the 2nd booster rollout will begin from Tuesday. The Government has not confirmed the eligibility criteria yet but we’re expecting to know this at the time of the announcement and current indications are that it will be at least the previously mentioned cohorts (65+, Māori and Pacific 50+, and immunocompromised).
We will update you with the confirmed criteria and key messages once the announcement is made.
Vaccine expiry dates, check the box – 21 June 2022
Vaccines can be used up until midnight of the expiry date printed on the box that the vaccine is provided in. This expiry date supersedes the expiry on the vaccine vial.
The vaccine expiry dates on the printed label on the vaccine box are calculated based on the time of removal from ultra-cold storage. These expiry dates and times must never be altered by vaccinators (this a restricted activity).
Recently Pfizer advised that the ultra-cold storage expiry date had been altered from its original printed date on the vial, extending it by 3 months for particular batches of vaccine. This did not alter the expiry date related to normal cold chain storage (+2 to +8˚C) at vaccination sites.
Due to this change in the ultra-cold storage expiry date there may be some instances where the date printed on the box does not match the vial expiry this is why it is essential to keep the vials with the box they come with and go off the expiry date on the box, not the vial.
Medsafe has provided consent for “9 month Comirnaty stability update”. This means that the shelf life moves from 6 months to 9 months (See here, 6.3 Shelf Life).
Important information regarding the consenting process – 21 June 2022
Informed consent is required for all vaccination events – this includes discussing possible reactions and what to do / where to go if these are experienced.
This is not being done in all situations. In some cases, this is due to not wanting to put the consumer off being vaccinated, or is being missed during second or booster doses because of an assumption that the consumer has already had this information.
A written post-vaccine handout (Getting your COVID-19 vaccine: What to expect) is recommended as part of the consenting process. Sites should ensure they have access to the latest leaflet and offer it at all vaccination events. You can request this collateral through romilly.smith@southerndhb.govt.nz
We recommend vaccinators watch the video on informed consent, myocarditis and pericarditis which is available free through the online IMAC e-learning page.
You and your staff may find it helpful during quieter times to role play the consenting process and other common scenarios to make sure everyone is comfortable with the required steps.
Second Booster update – 17 June 2022
We are expecting an announcement regarding a start date for second boosters in the next few weeks. Once the announcement is made, we may need to quickly pivot from our current activity levels to meet an upswing in demand.
What can you do now to make sure you're ready?
- Review and extend/renew your booking calendars past June 30 if you have not already done so - we are encouraging all providers to have a presence on the national booking system, Bookmyvaccine.co.nz
- Consider staffing and space requirements for the change in demand in your area
- Refresh all vaccinating staff with the 7 Rights of COVID-19 vaccine administration
- Ensure all vaccinating and clinical staff have logged into CIR in the past two months. If you have not, please request your access be requalified and reinstated by emailing shelby.davidson@southerndhb.govt.nz
- Review expected demand in your TA to understand when demand will peak (please see below)
- Review your enrolled patients and put in place plans to recall them if required
- Order vaccine, consumables and collateral as usual for now. In the event that the public announcement and a spike in demand can not be accommodated within your usual delivery schedule, we expect to be able to support you with out-of-schedule deliveries
- As always, get in touch with your project manager at Southern DHB or WellSouth manager if you have any questions or concerns
Forecast demand:
You will have seen TA level forecasting based on expected eligibility criteria in the webinar presentation on 9 June. We are expecting eligibility criteria to be extended and will let you know as soon as this is confirmed. In the meantime, we have reviewed the second booster demand by TA based on our understanding of eligibility extension, which can be downloaded here. Please note that these forecasts are only estimates but we hope they will assist with your planning.
Matariki opening hours – 17 June 2022
A reminder to all providers that if you are not open on Matariki, Friday 24 June, please make sure your bookings are closed off in BMV and your hours are updated on healthpoint.
Prescriptions and CIR – 16 June 2022
Some COVID vaccinations will require that a prescription is sighted and an electronic copy kept in CIR. A new function has been added to the CIR to remind vaccinators to upload a prescription where one is required.
A prescription from an authorised prescriber is required when a vaccine is being administered off-label in accordance with Section 25 of The Medicines Act 1981 (that is, when a Medsafe approved medicine is being used for an un-approved use) and the administration is not authorised via the Epidemic Preparedness (Medicines Act 1981 – COVID-19) Immediate Modification Order 2021 or other legislation. For example, third primary doses or early second boosters of the COVID-19 vaccine.
This guide will help vaccinators and administrators manage prescriptions in the Covid immunisation register: CIR - How to manage prescriptions in CIR
Reminder that vaccines cannot be self-prescribed - 15 June 2022
The Southern Vaccine Programme is tidying up incidents/adverse events in CIR (approx. 2000), and has identified a few instances where doctors or nurses have written their own prescriptions for vaccinations. We just wanted to take a moment to give a reminder that one shouldn’t self-prescribe, and that extends to vaccines.
Process for adverse events and incidents - 13 June 2022
Please find below a flow chart documenting the process to follow for adverse events/incidents, as outlined by the Ministry of Health. Please see the Incorrect Vaccination Incident Toolkit for definitions of adverse events and incidents.